Biography
Interests
CuhaWijay Sathiyajith* & Vijaya Rajendram
Neptune Bio-Innovations Pty Ltd, Unit E4 3-29 Birnie Avenue, Lidcombe, Australia
*Correspondence to: Dr. CuhaWijay Sathiyajith, Neptune Bio-Innovations Pty Ltd, Unit E4 3-29 Birnie Avenue, Lidcombe, Australia.
Copyright © 2019 Dr. CuhaWijay Sathiyajith, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Burgeoning interest have been directed in the recent past towards antibiotic resistance exhibited by bacterial infections. Currently, global unrestrained use of broad-spectrum antibiotics has been singled out as the prime factor for this devastating issue. Therefore, any efforts to mitigate usage of antibiotics in most common recurrent bacterial infections such as urinary and respiratory tract infections could be considered as a boon to circumvent this global challenge. In this article, we report the novel curcumin formulation composed of potassium citrate as urinary alkaliser along with bioavailable modified curcumin extract as the natural antibacterial agent. The in vitro evaluation of this unique composition proved more than log 5 reduction of E. coli viable bacterial colonies belonging to strain from ATCC25922 with 455μg/mL as the minimum bactericidal concentration (MBC). It was also established that the presence of potassium citrate in the composition does not alter the antibacterial efficacy of the bioavailable modified turmeric extract.
Abbreviations (if used)
a. Escherichia coli - E. Coli
b. Urinary Tract Infection (UTI)
c. Minimum Bactericidal Concentration - MBC
d. Minimum Inhibitory Concentration - MIC
e. Uropathogenic Escherichia coli - UPEC
f. Exopolysaccharides (EPS)
Introduction
The emerging trend of antibiotic resistance and efforts to circumvent this growing global challenge will soon
become the need of the hour.
Antibiotics are a class of medicine that treats and prevents bacterial infections. The coveted yet accidental discovery of Sir Alexander Fleming gave birth to the world’s first ever antibiotic, namely the “Penicillin” [1].
Ever since the successful treatment of Streptococus septicemea by penicillin, its discovery has led to easy treatment of fatal diseases, such as bacterial endocarditis, bacterial meningitis and pneumococcal pneumonia. More specifically, the impact on replacement of antisera and sulfonamides for the treatment of pneumonia rapidly paved way for screening of other natural products [2], which has led to a myriad of treatment options involving different antibiotics for several bacterial infections.
However, currently, the usage of antibiotics, or more specifically excessive usage of antibiotics, has opened doors for a cluster of issues termed as antibiotic resistance. The resistance to antibiotics occurs when bacteria changes its response to a specific antibiotic. A major cause of the issue is by actions of some physicians who throw a broad spectrum of antibiotics to combat a specific disease due to impracticality in identifying the infectious agent and prescribing an appropriate antibiotic within a day [2]. The approach has its pros and cons. Yes, while it saves lives, it also leads to cluster of negative issues. Antibiotic resistance not only results in higher medical costs and prolonged hospital stays, but also increased mortality as previously benign infections are turning to be malicious.
A plausible solution is to reduce the time between prevention of illness and providing an appropriate treatment. This could potentially lead to reduction in resistance and unnecessary prescription, perhaps provision of a chance of speedy recovery for needy patients. The methodology could be applied in the case of most common bacterial infections such as bacteriuria, which leads to the discussion on urinary tract infections.
Urinary tract infections are the most common form of bacterial infections. Globally, more than 150 million
people annually affected [3]. Clinically, UTIs are classified as uncomplicated and complicated UTIs.
Uncomplicated UTIs are prevalent in individuals who are otherwise healthy and have no structural or
neurological abnormalities in the Urinary tract, whereas complicated UTIs arises due to mitigation of
host defence mechanisms. Healthcare associated UTIs (HAUTI) and catheter associated UTIs are two
major categories of complicated UTI. Uncomplicated UTs are further classified as lower UTIs (cystitis)
and upper UTIs (pyelonephritis). Acute cystitis is the most common form of UTI in comparison to Acute
pyelonephritis, justified by the occurrence of 18-24 episodes of the acute cystitis per episode of pyelonephritis [3]. Furthermore, gender wise influence is demonstrated by most common occurrence of uncomplicated
cystitis in women in comparison to men where complicated forms of UTI are prevalent.
UTIs can be caused by both Gram-negative and Gram-positive bacteria, as well as by certain fungi. The most common causative agent for both uncomplicated and complicated UTI is the uropathogenic Escherichia coli (UPEC) [4]. The prevalence of UPEC in uncomplicated cystitis is followed by Klebsiella pneumoniae, Staphylococcus saprophyticus, Enterococcus faecalis, group B Streptococcus (GBS) and Proteus mirabilis (Fig. 1).
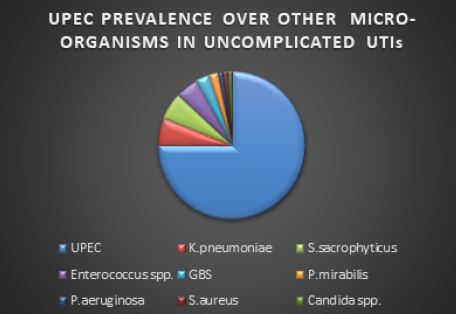
Most typically, UTIs are initiated with periurethral contamination by a UPEC residing in the gut and
subsequent colonization of the urethra and will be followed by migration of the pathogen to the bladder, an
event that requires appendages such as flagella and pili. In the bladder, the consequences of complex hostpathogen
interactions ultimately determine whether UPEC will be successful in colonization or eliminated.
Multiple bacterial adhesins recognize receptors on the bladder epithelium (i.e. uroepithelium) mediate
colonization [5]. Ascension does not halt there, indeed through multiplication and by overcoming host
immune surveillance the uropathogens can subsequently ascend to the kidneys, wherein they colonize the
renal epithelium. Furthermore, uropathogens may cross the tubular epithelial barrier to reach the blood
stream, culminating in bacteraemia [5].
UPEC colonization during Uncomplicated urinary tract infection (UTI) is characterized by dysuria (pain during urination) and urinary frequency (very frequent urination and uncontrollable urge to void the bladder) [6]. Most common intervention for patients suffering from symptomatic uncomplicated UTI is the antibiotics. However, long term use of such antibiotic treatment option inevitably leads to long-term alteration of the normal micro-biome residing in vagina, gastro-intestinal tract and may even lead to multi drug resistant bacterial strains. The development of resistance mainly prevalent in extended spectrum beta lactamase secreting enzymes.
Currently, antibiotics such as trimethoprim sulfamethoxazole, ciprofloxacin and ampicillin are the most commonly recommended therapeutics for UTIs [7]. However, continuous rise in antibiotic resistance (Fig. 2) and high recurrence rates virtually left us with very few effective first-line treatment options, such as cephalosporin and carbapenems [8].
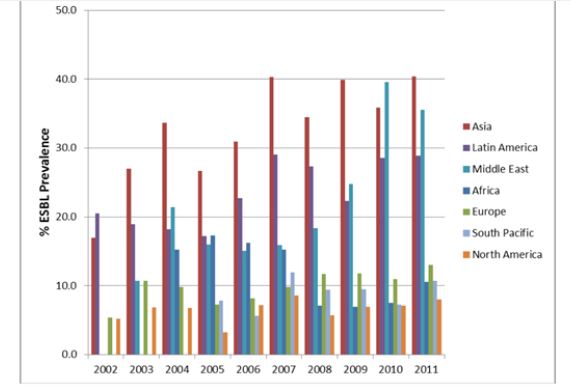
However, with the knowledge of basic biology of UTI pathogenesis to specifically target virulence pathways, many promising approaches are being developed. One could envisage these anti-virulence therapeutics should theoretically enable us to effectively neutralize, or ‘disarm’, the capacity of UTI pathogens to cause disease, without altering the gut commensal microbiome.
Furthermore, majority of the therapeutic or prophylactic interventions are broadly based on two-faced strategy, namely, disperse the preformed biofilm and/or inhibit the formation of new biofilm [9]. The strategy could potentially provide solution for antibiotic resistance by reduction in duration between prevention of illness and providing an appropriate treatment. This could potentially lead to reduction in resistance and unnecessary prescription, perhaps provision of a chance of speedy recovery for needy patients.
As the focus of the article is not on urinary tract infections, but rather an introduction to a plausible solution for it, interested readers are requested to read elsewhere. Briefly, urinary tract infections (lower region) referred to infection of the bladder and the urethra, known as cystitis and urethritis, respectively.
E. coli bacterial transfer from faecal residue through the rectum to bladder has been facilitated by the proximity of rectum and bladder in women. These infections yield an acidic urine which leads to a burning sensation during urination. The most common approach is to provide symptomatic relief by giving potassium citrate [10]. Potassium citrate alkalize the urine by enhancing plasma bicarbonate concentration due to its metabolic conversion in vivo.
As the central science, Chemistry equally plays a major role in the development of antibiofilm agents in
conjunction with genetic engineering. A number of chemical moieties including [11]. In order to provide
a glimpse on the mechanism of preventing biofilm formation, it is necessary to understand the structure
of a biofilm. The matrix component of a biofilm consists of exopolysaccharides, or some time known as
exopolymeric substances (EPS), are secreted by bacteria to their surroundings. These exopolymeric substances
promotes adhesion and colonisation of bacterial cells. EPS, therefore, acts as a safety net for the growth and
activity of bacterial cells within a biofilm.
One could envisage biomimetic supramolecular designs that prevent adhesion could be a good starting point. Indeed, several successful in vitro and in vivo studies have been reported on this supramolecular chemistry approach [12].
In fact, resolving antibiotic resistance caused by biofilm formation could be approached from many angles and supramolecular chemistry approach is just only one effective way of resolving the issue.
Along these lines, therapeutic interventions based on natural and synthetic origin have been evaluated as alternative therapies to antibiotics. More specifically flavonoids, a class of natural product has been widely investigated [13]. Infact the current treatment for uncomplicated lower UTIs based on Cranberries is founded on proanthocyanidins, a class of flavonoids acting against adhesion of bacterial cells and thereby preventing biofilm formation to some extent. Furthermore, therapeutic interventions based on cranberries and other similar treatment options provide only prophylactic benefit by providing symptomatic relief. Moreover, they hardly provide a lasting solution for recurrence of uncomplicated UTIs.
Taking in to consideration of above facts, investigators of Neptune Bio-innovations (NBI), Australia, envisaged that if they could find a natural flavonoid equivalent that could both destroy the preformed biofilm while acting as a “firewall” for new biofilm formation would then provide a solid foundation towards more lasting solution for patients undergoing UTI discomfort. Prompted by the marketing hype on turmeric and also backed by literature evidence [14], investigators of NBI focused their attention on curcuminoids, a class of polyphenolic compounds and initially investigated its antibactericidal properties by utilising turbidity assays based on minimum inhibitory concentration based on standard protocols.
The pre-liminary research gave confidence and inspiration to dive deeper, more specifically to carry out minimum bactericidal concentration (MBC) assays, the result was the astonishing finding that curcumins able to provide greater than log 5 reduction of bacterial colonies with just 0.016uM concentration, as obtained from regression analysis of the assay. Its noteworthy that presence of greater than 100,000 colonies per milliliter of urine is reported as significant bacteriuria [15].
Materials and Methods
Distilled water
Tryptone Soya Agar (TSA) Oxoid CM131
0.1% Peptone
Nutrient Broth 2.2% Lecithin & 3% tween
Standard Hard Water
Ratek VM 1 vortex 2700rpm
Preparation of Standard Hard Water
Each of the prepared dilutions was pre-warmed in a 37°C incubator for 30 minutes until inoculation of the
culture. Each dilution was inoculated with 0.05ml of E. coli ATCC 25922 prepared at a concentration of
5x109cfu/ml and vortexed for 10 seconds at 2,700rpm. The inoculated samples were then placed back into
the 37°C incubator for 24 hours contact time. At the end of the 24-hour incubation, 4.5ml of NBLTW
neutralizer was added to each test matrix and vortexed for 10 seconds at 2,700rpm. This was considered the
10-1 dilution.
Serial dilutions of the neutralized matrix were prepared by taking 1ml of the neutralized sample and dispensing into 9ml of 0.1% peptone water to obtain the 10-2 dilution. This process was repeated another three times to obtain dilutions from 10-1 to 10-5.
From each of the prepared dilutions 2ml was taken and plated 1ml in duplicate for each dilution in sterile labelled petri dishes. The plates were then poured with 15ml of TSA.
The plates were dried for 15 minutes and incubated at 37°C for 48 hours.
Dissolve 19.84g magnesium chloride (MgCl2) and 46.24g calcium chloride (CaCl2) in water and dilute to
1000ml. Sterilize in the autoclave.
Dissolve 35.02g sodium bicarbonate (NaHCO3) in water and dilute to 1000ml. Sterilize by membrane
filtration.
Placed approximately 650mL of BSEN-SHW in a 1000mL volumetric flask and add 6,0mL of solution
A, then 8,0ml of solution B. Mix and dilute to 1000mL with water. The pH of the hard water shall be 7,0
± 0,2, when measured at (20 ± 1)°C as per BSEN and the pH was adjusted accordingly, using 1M HCl or
1M NaOH.
25g of Oxoid Nutrient Broth 2 Powder Base (CM67) with the addition of 130g of Lecithin and 30g of
Tween 80 was made up to 1000ml with distilled water. Dispensed into Macs and then autoclaved at 121°C
for 15 minutes.
Colonies are taken from a plate prepared with E. coli and placed into 10ml of 0.1% Peptone containing glass
beads. Enough colonies were taken to produce a culture in the range of 5-10x109cfu/ml.
UC-IC50-2 was prepared by blending modified bioavailable turmeric extract and potassium citrate
monohydrate in a certain ratio.
Accurately weighed 3.00g of UC-IC50-2 was added to an Eppendorf labelled as UC-IC50-2-Stock A and
was dissolved in 1.50mL of BSEN Standard Hard Water (BSEN-SHW) as per the instructions provided
by Neptune Bio-innovations and vortexed until completely dissolved.
The 200% stock solution was then used to prepare the following serial dilutions:
0.25ml of UC-IC50-2-Stock A was diluted with 0.25ml of BSEN SHW and vortexed for 10 seconds at
2,700rpm to produce UC-IC50-2-Working Stock B (100%) and labelled as UC-IC50-2-WSB_100.
0.225ml of the UC-IC50-2- Stock A solution was diluted with 0.275ml of BSEN SHW and vortexed
for 10 seconds at 2,700rpm to produce UC-IC50-2-Working Stock B (90%) and labelled as UC-IC50-2-
WSB_90.
0.200ml of the UC-IC50-2-Stock A solution was diluted with 0.300 ml of BSEN SHW and vortexed
for 10 seconds at 2,700rpm to produce UC-IC50-2-Working Stock B (80%) and labelled as UC-IC50-2-
WSB_80.
0.175 ml of the UC-IC50-2-Stock A solution was diluted with 0.325 ml of BSEN SHW and vortexed
for 10 seconds at 2,700rpm to produce UC-IC50-2-Working Stock B (70%) and labelled as UC-IC50-2-
WSB_70.
0.15 ml of the UC-IC50-2-Stock A solution was diluted with 0.350 ml of BSEN SHW and vortexed for
10 seconds at 2,700rpm to produce UC-IC50-2-Working Stock B (60%) and labelled as UC-IC50-2-
WSB_60.
0.125 ml of the UC-IC50-2-Stock A solution was diluted with 0.375 ml of BSEN SHW and vortexed
for 10 seconds at 2,700rpm to produce UC-IC50-2-Working Stock B (50%) and labelled as UC-IC50-2-
WSB_50.
0.100 ml of the UC-IC50-2-Stock A solution was diluted with 0.400 ml of BSEN SHW and vortexed
for 10 seconds at 2,700rpm to produce UC-IC50-2-Working Stock B (40%) and labelled as UC-IC50-2-
WSB_40.
0.075ml of the UC-IC50-2-Stock A solution was diluted with 0.425ml of BSEN SHW and vortexed for
10 seconds at 2,700rpm to produce UC-IC50-2-Working Stock B (30%) and labelled as UC-IC50-2-
WSB_30.
0.050ml of the UC-IC50-2-Stock A solution was diluted with 0.450ml of BSEN SHW and vortexed for
10 seconds at 2,700rpm to produce UC-IC50-2-Working Stock B (20%) and labelled as UC-IC50-2-
WSB_20.
0.025ml of the UC-IC50-2-Stock A solution was diluted with 0.475ml of BSEN SHW and vortexed for
10 seconds at 2,700rpm to produce UC-IC50-2-Working Stock B (10%) and labelled as UC-IC50-2-
WSB_10.
Results
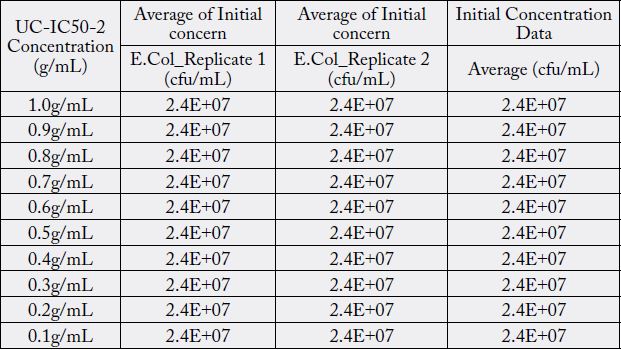
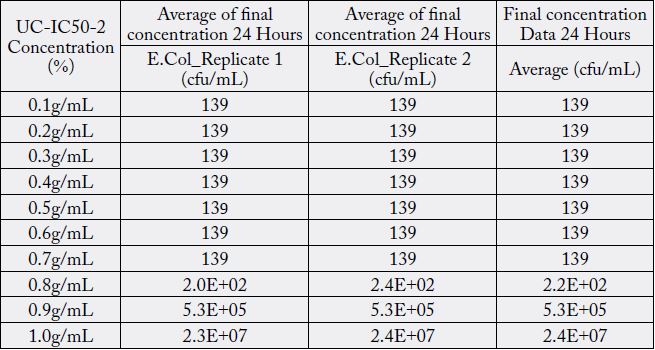
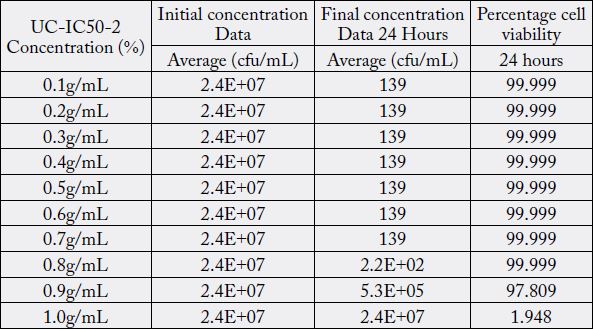
Discussions
A wide variety of methods have been in practice to evaluate the efficacy of antimicrobial activity of medicinal
herbal extracts, agar-disk diffusion, ATP bioluminescence agar or broth dilution to name but a few [16].
However, dilution methods based on agar or broth are effective both in their efficacy to evaluate minimum
inhibitory concentration (MIC) of the antibacterial agent as well as from cost perspective. Indeed, broth
micro or macro dilution methods are most widely practiced antimicrobial susceptibility testing. However,
inoculum size, type of growth medium and incubation time and diversity in the preparation method could
influence MIC values.
The minimum inhibitory concentration (MIC value) recorded is defined as the lowest concentration of the assayed antimicrobial agent that inhibits the visible growth of the microorganism in question. Wherein, minimum bactericidal concentration (MBC) is defined as the lowest concentration of the assayed antimicrobial agent that is required to kill 99.9% of the specific bacterial strain. A series of dilutions of UCIC50- 2 was prepared and was subjected antibacterial kill rate assays as described in the preceding section. A precise evaluation of MBC was achieved by obtaining Dose-Response curve using GraphPad-Prism.
Furthermore, minimum bactericidal concentration of 455μg/ml obtained in this study is in line with the investigative reports published elsewhere [16]. However, antibacterial properties of Curcuma longa in its native form or as derivatives in conjunction with potassium citrate in the same formulation has never been explored. Furthermore, antibacterial properties of curcuminoids has not been affected by the presence of alkaliser. Ten serial dilutions have been considered to ensure that there are enough assay concentrations having responses on the lower and upper plateaus of the dose-response curve (Fig. 3).
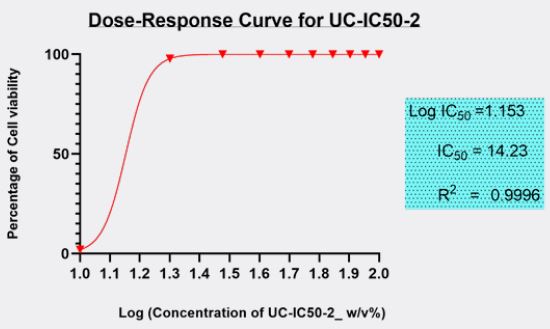
Therefore, we have been prompted to opt out a standardized protocol established by clinical and laboratory standards institute (CLSI) or BESN. The results have been investigated independently from three laboratories and were confirmed with similar results with same standardized protocols.
Essentially, there are other bacterial strains have been identified as causative agents for UTIs [4]. Although this study had focused on the most probable bacterial strain causing uncomplicated UTI, namely ATCC25922, it will be equally interesting to investigate other types of bacterial strains and related therapeutic potential of this formulation. Moreover, the therapeutic efficacy of this formulation could be investigated for other type of non-serious diseases caused by ATCC25922.
Conclusions
We observed reduction of greater than log 5 in the viable E. Coli colonies with just 0.016 micro molar
concentration of the active, obtained from regression analysis of the assay. The investigators of NBI strongly
believe that the mechanism of action is based on “apoptosis like” killing of bacteria. Currently, the hypothesis
has been well established as a scientific fact through an in-depth study published in PLOS One, utilizing
the specific strain used in NBI investigator’s assay [17]. On the other hand, incorporation of potassium
citrate along with curcumins (provided from a modified bioavailable turmeric extract) does not prevent the
antibactericidal property of curcumins and does not contribute to any pH variation whatsoever in solution,
as proved in long term stability studies though clinical therapeutic efficacy is yet to be established.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!