Biography
Interests
Aloy-Amadi Oluchi, C.1, Amilo Grace, I.1, Ifeanyichukwu Martin1, Obeagu Emmanuel Ifeanyi2*, Ken- Ezihuo Stella3 & Ahaneku Osuji4
1Department of Medical Laboratory Science, Nnamdi Azikiwe University, Nnewi Campus, Anambra, Nigeria
2Medical Laboratory Science, University Health Services, Michael Okpara University of Agriculture, Umudike,
Abia State, Nigeria
3Department of Medical Laboratory Science, Rivers State University, Port Harcourt, Nigeria
4Department of Microbiology and Immunology, University of Abuja Teaching Hospital, Gwagwalada Abuja,
Nigeria
*Correspondence to: Obeagu Emmanuel Ifeanyi, Department of Medical Laboratory Science, University Health Services, Michael Okpara University of Agriculture, Umudike, Abia State, Nigeria.
Copyright © 2019 Obeagu Emmanuel Ifeanyi, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Normal Pregnancy is characterized by changes in Immunological profiles which have been linked
to physiological adaptation. These immunological parameters are either elevated or decreased to
reinforce immunity and maintain a healthy pregnancy, but returns to that of non-pregnant state at
about 4-6 weeks after delivery.
The study was undertaken to determine the concentrations of some anti-inflammatory and proinflammatory
cytokines in pregnancy at different trimesters and to compare these non-pregnant
females.
The research involved 160 apparently healthy pregnant women attending antenatal clinic at
Nnamdi Azikiwe University teaching Hospital, Nnewi, Anambra State Nigeria, at booking in their
first trimesters. Five Milliliters (5ml) of Venous blood was collected from each subject, of which
4mls was put into gel tubes for Screening for HIV I & II, Hepatitis B Surface Antigen(HBsAg),
Hepatitis C virus(HCV), Veneral Disease Research Laboratory (VDRL) and cytokines while 1ml
was aliquoted into potassium EDTA anti coagulated tubes for malaria parasite screening using
standard laboratory techniques. These women were followed - up till the last trimester. During the
second trimester, 156 pregnant women were eligible, and only 140 subjects completed the study
during the third trimester.
In pregnant women, 1L-4{pg/ml}, first trimester {28.12+17.38}, second {31-33 +17-51} and third
{33.81+ 17.78} were significantly increased compared to controls {27.73 + 21.68}. 1L-10 {pg/
ml}. first trimester {30.54+ 13.10} second {34.54 + 16.41} and third {38.66 + 22.89} were also
significantly increased than controls {26.62 + 76.61} 1L-2 {pg/ml},first trimester {52.18 + 31.70}
second {48.58+ 31.01},and third {46.82+31.13}were significantly decreased compared to controls
(56.17+ 31.45) while TNF-α (ng/l), first trimester (168.97 + 126.33),second (166.69+ 67.43) and
third (165.95 + 68.97) trimesters showed no significant difference compared to controls (169.27
+56.63) when compared among the trimesters, 1L-4 in first trimesters (28.12+ 17.38), second
(31.33 + 17.51) and third (33.81 + 17.78) were significantly increased. 1L-10 (30.54 + 13.10)
(34.54 +16.41) and (38.66 + 22.89) were significantly decreased, while TNF-α (168.97 + 126.33),
(166.69 + 67.43) and (165.94 + 68.97), showed no difference.
The findings showed that anti- Inflammatory and Pro-inflammatory cytokines alter remarkably in
pregnancy. Therefore, their assessment is strongly advocated in the prediction of gestation prognosis.
Introduction
Pregnancy also known as gestation is the time during which one or more offspring develops inside a woman
[1]. Pregnancy can occur by sexual intercourse or assisted reproductive technology [2].
Childbirth typically occurs around 40 weeks from the last menstrual period (LMP) [1,3]. Pregnancy is typically divided into three trimesters. The First trimester is from week one through 12 and includes conception, which is when the sperm fertilizes the egg. The second trimester is from week 13 through 28, while the third trimester is from 29 weeks through 40 weeks [1]. Pregnancy is a physiologically immune compromised state, during which alterations in T- lymphocyte subsets may occur [4]. It requires physiologic adaptations in all maternal systems including the immune system. Recent investigations have shown that during pregnancy, maternal circulating immune cells undergo modifications in cell counts, phenotypes, functions and ability to produce soluble factors, such as cytokines. The ultimate goal is to establish and maintain a successful pregnancy which involves a state of selective immune tolerance, immune suppression and strong anti- microbial immunity. The mammalian immune system has evolved to co-exist with these needs by down-regulating potentially dangerous T-cell-mediated immune responses, while activating certain components of the innate immune system, such as monocyte and neutrophils [5].
Cytokines are soluble proteins synthesized by various cells of the immune system and which act as immune regulators. Normal pregnancy is associated with changes in pro-inflammatory and anti-inflammatory cytokines [6]. It is accompanied by decreased production of type 1 Pro-inflammatory cytokines and increased production of type 2 anti-inflammatory cytokines [6,7]. The anti - inflammatory cytokine are a series of immune regulatory molecules that control the pro-inflammatory cytokine response. Cytokines act in concert with specific cytokine inhibitors and soluble receptors to regulate the human immune response. Their physiologic role in inflammatory and pathologic role in systemic inflammatory states are increasingly recognized [8]. IL- 4 and IL-10 are examples of major anti - inflammatory cytokines. The functional definition of an anti - inflammatory cytokines is the ability of the cytokines to inhibit the synthesis of IL -1, TNF -α and other major pro-inflammatory cytokines. For example, IL-4 promotes the lymphocyte development, inhibition of lipopolysaccharide (LPS) -induced pro-inflammatory cytokine synthesis, while IL-10 inhibits monocyte, macrophage and neutrophils. Anti-Inflammatory cytokines perform a multitude of functions during normal pregnancy by promoting placental formation, modulating trophoblast invasion and differentiation, inducing placental proliferation and angiogenesis and inhibiting pro-inflammatory cytokines [8]. IL- 4 production is increased in the gravid state and levels of IL- 4 increase throughout normal pregnancy [9]. Progesterone is a known inducer of IL-4 and together they act to inhibit Th1 responses during pregnancy (Svensson et al., 2001). IL-4 and IL-10 increase in successful pregnancy with a type-1 - to - 2 shift characterizing the third trimester [9]. They play crucial roles in the success of pregnancy and there is strong evidence that a deficiency in IL-4 and /or IL- 10 contributes to infertility, spontaneous abortion, pre-term birth (PTB), fetal growth rejection (FGR) and hypertensive disorders in pregnancy (Chatterjee et al., 2014). IL - 10 up- regulates its own production by signling between immune cells and also suppression of both immune and non-immune cells [10].
Pro - Inflammatory cytokine is a type of cytokine (signaling Molecule) that is excreted from the immune cells and certain other cell types that promote inflammation. They are produced predominantly by activated macrophages and are involved in the up - regulation of inflammatory reactions, e.g. IL-β, IL-2, IL-6 and TNF- α are examples of pro-inflammatory cytokines [11]. Normal pregnancy is accompanied by decreased production of type 1 pro-inflammatory cytokines and increased production of type - 2 anti-inflammatory cytokines [6,7]. IL - 2 pattern of cytokine secretion associated with a T-helper 1(Th1) immune response [12]. Over-expression of IL - 2 inhibits pregnancy viability [13]. Women whose conceptions end in abortion have significantly high IL-2 serum levels. Elevated IL-2 serum concentrations have been found during the first trimester in women who later developed pre-eclampsia. IL-2 production decreases in physiologic human pregnancy and also decreased in all trimester of pregnancy compared with non-pregnancy controls. It is increased in pathologic conditions [9].
TNF-α is produced by the placental trophoblast cells and feto-placental macrophages, thus up - regulating the endothelial expression of platelet derived growth - factor, endothelial - 1 and the plasminogen activator inhibitor [14]. It is a type - 1 cytokine and is implicated for pregnancy failure when the concentration is raised during pregnancy [15]. TNF-α presents a stable production profile in all stages of pregnancy [16]. As pregnancy develops high TNF- α concentration have been related to the development of pre-eclampsia and gestational diabetes mellitus (GDM) [17].
The study was undertaken to determine the effect of pregnancy on some anti-inflammatory and proinflammatory cytokines in pregnant women at NnamdiAzikiwe University Teaching HospitalNnewi (NAUTH), Anambra State, Nigeria.
Materials and Methods
The Research was conducted at NAUTH, Nnewi, Anambra State between Jan - Dec 2016.
One Hundred and Sixty (160) apparently pregnant women who presented for booking for antenatal care in
their first trimester (3rd month) visit at NAUTH, Nnewi were enrolled for the study after ethical committee
of NAUTH and written informed consent were obtained. Their age range was between obtained. Their age
range was between 20 - 40 years. Similarly 160 age matched non-pregnancy females consisting of health
science students and the staff of NAUTH served as controls.
Methodology
This was a longitudinal study carried out at the antenatal care clinic of NAUTH Nnewi, Anambra State
during the period of January to December, 2016. All pregnancy women who presented for booking for
antenatal care in their first trimester (3rd Month) visit at NAUTH, NNEWI were recruited for the study.
They were enrolled after providing their informed concert. Questionnaires were administered to obtained
their medical and obstetrics history (age, parity, gestation age etc). Pregnancy and its duration were confirmed
by ultrasound scan, and pregnant women with multiple pregnancy were excluded.
During the first trimester visit and at subsequent trimesters, the blood pressure was measured using a sphygmomanometer. The weight and height measured were used to calculate the body mass index (BM1), which was expressed as weight (kg)/Height (m2). Five milliliters (5mls) of blood was collected from each subject by means of hypodermic syringe and needle, 4mls into gel tubes for screening HIV I & II, Hepatitis B surface antigen A(HBsAg), Hepatitis C virus (HCV), Veneral Disease Research Laboratory and Cytokines.1ml was aliquoted into potassium EDTA for malaria parasite screening.
At the end of the screening exercise, pregnant women who were not eligible were excluded. One hundred and Sixty (160) apparently healthy pregnant women were enrolled in the research as the study group. Similarly, 160 age- matched non-pregnant females consisting of health science students and the staff of NAUTH, who served as controls were enrolled for the study based on the inclusion criteria. Pregnancy test was conducted on the non-pregnant female to confirm they were not pregnant. These pregnant women were on iron supplements and were followed-up till the last trimester - second trimester {5th month} and third (8th month) The same tests were conducted on the non- pregnant control samples at NAUTH laboratories. At the second trimester, 156 pregnant women were followed - up. The remaining 4 miscarried or changed address. During the third trimester, only 140 pregnant women completed the study. Those who could not complete the survey were ineligible because they had still birth, miscarried, changed address or felt the survey was a disturbance to them. Results from pregnant subjects were compared with that of controls and comparisons were also made among the trimesters.
The research study was approved by the Ethics Review committee of NnamdiAzikiwe University Teaching
Hospital (NAUTH), Nnewi, and informed consent was obtained in writing before reality each subject the
study.
Statistical analysis was done using students unpaired two tailed t-test to determine whether a parameter from
two different groups differ significantly or not. All comparisons were performed using one - way analysis
of variance (ANOVA) to compare the means of three or more groups to determine if they are significantly
different. Pearson’s correlation analysis was undertaken to access the statistical relationships or associations
among the variable according to trimesters and statistical significance was calculated using post hoc test to
analyses the results of the experimental data. Differences were considered to be significant at p < 0.05.
Results
Table 1 shows levels of anti-inflammatory and pro-inflammatory cytokines in non-pregnant females
(controls), first, second and third trimesters (Mean ± SD).
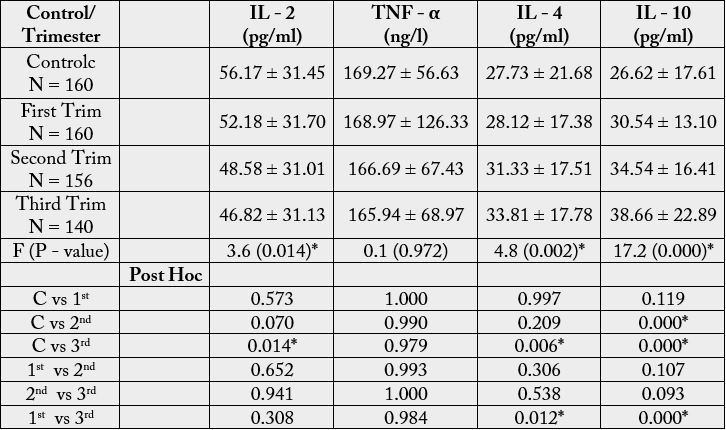
Key:
Mean difference is significant at p< 0.05*
C = Control
SD = Standard Deviation
Trim = Trimester
The mean levels of IL - 2 in first (52.18 + 31.70), second (48.58 + 31.01) and third (46.82 + 31.13) trimesters and control (56.17 + 31.45) were significantly different (F = 3.6, P = 0.014). On the other hand, TNF - α mean values in control (169.27 + 56.63) and first (168.97 +126.33), second (166.69 + 67.43) and third (165.94 + 68.97) trimesters, showed no difference (F = 0.1, P = 0.972). IL - 4 with mean levels in first (28.12 + 17.38). Second (31.33+ 17.51), third (33.81 + 17.78) and control (27.73 + 21.68) were significantly different. (F = 4.8, P = 0.002). The mean values of IL - 10 in control (26.62 + 17.61), first (30.54 + 13.10), second (34.54 + 16.41), and third (38.66 + 22.89) were different significantly (F = 17.2, P = 0.000).
The mean of IL - 2 in first trimester (52.18 + 31.70), showed no difference compared with the control
(56.17 + 31.45) (P = 0.573). That of the second trimester (48.58 + 31.01) showed no significant difference
compared with the control (56.17 + 31.45) (P = 0.070), while the third trimester ( 46.82 + 31.13) showed
a significant difference compared with the control (56.17 + 31.45) (P = 0.014) the mean level of IL - 2 in
the first trimester (56.17 + 31.45) showed no difference compared with the second (48.58 + 31.01) (P =
0.652), while that between the second (48.58 + 31.01) and third (46.82 + 31.13) trimesters also showed no
significant difference (P = 0.941). Similarly, that of first trimester (52.18 + 31.70) showed no significant
difference compared with the third (46.82 + 31.13) (P = 0.308). The mean values of TNF - α in the first
trimester (168.97 + 126.33) was not significantly different from the control (169.27 + 56.63) (P = 1.000).
There was no significant difference in the mean levels of TNF - α in the second trimester (166.69 + 67.43)
compared with the control (169.27 + 56.63) (P = 0.990). That of the third trimester (165.94+ 68.97), also
showed no significant difference compared with the control (169.27 + 56.63) (p = 0.979). The mean levels of TNF - α in the first trimester (168.97 + 126.33) showed no significant difference compared with the second
(166.69 + 67.43) (P = 0.993). There was no significant difference in TNF - α in the second (166.69 ± 67.43)
trimester compared with the third (165.94 + 68.97) (P = 1.000), and no significant difference in the first
(168.97 + 126.33) compared with the third (165.94 + 68.97) (P = 0.984).
IL - 4 showed no significant difference in the control (27.73 + 21.68) compared with the first (28.12 + 17.38) (P = 0.997) and second (31.33 + 17.51) trimesters, (P = 0.209), respectively, and a significant difference compared with the third (33.81 + 17.78) (P = 0.006). There was no significant difference in the mean levels of IL - 4 in first trimester (28.12 + 17.38) and second (31.33 + 17.51) (P = 0.306) and between second (31.33 + 17.51) and third trimester (33.81 + 17.78) (P = 0.538), while a significant difference was observed in the first trimester (28.12 + 17.38) and trimester (33.8 + 17.78) (P = 0.012).
The mean level of IL - 10 in the control (26.62 + 17.61) showed no significant difference compared with the first trimester (30.54 +13.10) (P = 0.119), and a significant difference was observed when compared with the second (34.54 + 16.41) (P = 0.000) and third (38.66 + 22.89) (P = 0.000), respectively. There was no significant difference in the mean levels in the first trimester (30.54 + 13.10) compared with the second (34.54 + 16.4) (P = 0.107) and between the second (34.54 + 16.41) and the third trimester (38.66± 22.89) (P = 0.093), but a significant difference was observed between the first trimester (30.54 + 13.10) and the third (38.66 + 22.89) (P = 0.000).
Tables 2 shows levels of anti - and pro- inflammatory cytokines in pregnant women at different trimesters (Mean + SD).
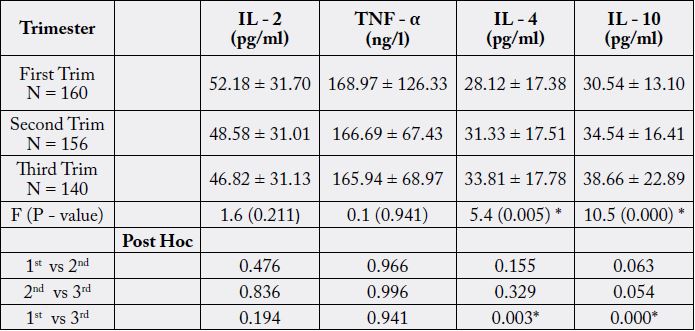
Key:
* Significant at P < 0.05
Trim = Trimester
The mean levels of IL - 2 in the first (52.18 + 31.70), second (48.58 + 31.01) and third (46.82 + 31.13) were not significantly different (F = 1.6, P = 0.211). There was also no significant difference in the mean levels of TNF - α in the first (168.97+126.33), second (166.69+67.43) and third (165.94 +68.97) trimesters (F=0.1, P = 0.941). IL – 4 showed a significant difference in the first (28.12 + 17.38), second (31.33+17.51), and third (33.81+17.78) trimesters respectively (F = 5.4, P = 0.005), A significant difference was also observed in IL - 10 in the first (30.54+13.10), second (34.54+16.41), and third (38.66+22.89) (F = 10.5, P = 0.000).
There was no significant difference in IL- 2 in the first trimester (52.18 ± 31.70) compared with the second
(48.58± 31.01) (P = 0.476). No difference was observed between the second trimester (48.58 ± 31.01) and
third (46.82 ± 31.13) (P=0.836). There was also no significant difference when the first trimester (52.18 ±
31.70) was compared with the third (46.82 ± 31.13) (P=0.194). TNF - α showed no difference when the
first trimester (168.97 ± 126.33) was compared with the second (166.69 ± 67.43) (P=0.966), second (166.69
± 67.43) compared with the third (165.94± 68.97) (0.996), and first (168.97 ±126.33) compared with the
third (165.94 ± 68.97) (P=0.941).
IL - 4 showed no significant difference when the first trimester (28.12 ±17.38) was compared with the second (31.33 ± 17.51) (P =0.155), second (31.33 ± 17.51) compared with the third (33.81 ± 17.78) (0.329), but a significant difference was observed when the first trimester (28.12 ±17.38) was compared with the third (33.81 ± 17.78)) (0.003). There was no significant difference in IL - 10 when the second trimester (34.54 ± 16.41) was compared with the first (30.54 ± 13.10) (P = 0.063) and third (38.66± 22.89) (P = 0.054), while a significant difference was observed when the first (30.54 ± 13.10) was compared with the third (38.66 ± 22.89) (P = 0.000).
Discussion
Normal pregnancy is associated with changes in immunological parameters (chattered et al., 2014). In
recent past, maternal mortality has been remarkably attributed to pregnancy- related conditions. Most
complications in pregnancy are associated with certain immunological dysfunctions. The consistency of
this distribution has scarcely been studied in our environment for various gestational periods of pregnancy.
Therefore, this necessitated this study, as the findings will encourage early evaluation of cytokines and
monitoring of pregnancy, in order to predict the outcome.
In this study the levels of four differ cytokines during physiologic pregnancy were determined. IL - 4 was significantly increased in pregnancy as compared to the non-pregnant state. This agrees with the work done by Marzi et al., 1996 [9], who showed that IL - 4 production is increased in the gravid state and that a successful pregnancy is characterized by increased production of Th2 cytokines e.g. IL - 4. Jones et al., (2000), also stated that normal pregnancy is accompanied by an increased production of type 2 antiinflammatory cytokines.
The increase in IL - 4 in pregnancy may be due to the fact that pregnancy is anti-inflammatory condition and a shift would lead to pregnancy complications (Chatterjee et al., 2014). IL - 4 being a Th2 Cytokines, is beneficial for pregnancy, promoting proliferation and differentiation of unit, inhibiting the production of
Th1 cytokine. Hence successful pregnancy is associated with preferential development of the Th2 profile. The increase in the production of hormones e.g. progesterone during pregnancy is also part of it. Progesterone is a known inducer of IL - 4. It enhances IL 2 production by human T - cells and together they act to inhibit Th1 Reponses during pregnancy (Svensson et al., 2001). IL - 4 being a Th2 - type cytokine, is produced by the deciduas and are required for embryo implantation and development. Hence, the increase in pregnancy more than non-pregnancy state.
This study showed a progressive increase in IL - 4 from the first to the third trimester. This is similar to the work done by Marzi et al., 1996 [9] and Ekerefelt et al., 1997 who stated that IL - 4 showed a constant presence at the first two trimester and the highest quantities were observed in the third trimester, when the concentration of progesterone is at its highest. The second phase of pregnancy is an anti-inflammatory state, so IL -4 is increased. This stage is a period of rapid fetal growth and development. The mother, placenta, and fetus are symbolic, and the predominant immunological feature is induction of anti- inflammatory state.
Interleukin 10 being an anti -inflammatory cytokines or a Th2 - type cytokine was high in pregnancy compared with their non-pregnancy counterpart. This agrees with the study carried out by Chatterjee et al., 2014, who showed that IL - 10 production increased in pregnancy than in non-pregnant state. Abdolreza et al., 2011 [7] also stated that during pregnancy, IL - 10 levels were increased than in non-pregnancy state. Increased in IL-10 in pregnant women was also observed by Holmes et al., 2003. There was increase IL- 10 levels from first to the third trimester, and this also agrees with Abdolreza et al., 2011 [7], who stated that during pregnancy, IL - 10 levels were increased with increase in gestational age and together with IL - 4 play crucial role in the success of pregnancy (Chatterjee et al., 2014). It agrees with the work done by Denney et al., 2011, who showed an overall increase overall - regulatory in counter - regulatory cytokines e.g. IL - 10 as pregnancy progresses. (Chatterjee et al., 2004), stated that normal pregnancy was determined to have increased IL- 10 production during the first and second trimesters but, not in the third trimester and decreases prior to labour and delivery of the fetus and placenta, but increases post labour. They play protective roles during pregnancy and being Th2 - type cytokines are increased during pregnancy. In normal pregnancy, the secretion of IL - 10 assists the maintenance of a less pro - inflammatory environment, favoring a more regulated immune micro environment that is opposite to the presence of a fetus. The balance of maternal immune response controlling the inflammatory mechanism is dependent on IL - 10. Regulatory features of IL - 10 (Pleomorphic cytokine) in the immune stimulatory and immunosuppressive activity might be associated with the regulation of the Th1 - Th2 activities (Denney et al., 2011). Normal Progesterone, the concentration of which increases in pregnancy favours the production of IL - 10. This accounts for the observation that the highest quantities of IL - 4 and IL - 10 were observed in the third trimester of pregnancy, when the concentration of progesterone is at its highest.
In this study, IL - 2 was decreased in pregnant females as compared to non-pregnant ones. This agrees with the work done by Marzi et al., 1996 [9] who stated that IL - 2 production decreases in physiologic human pregnancy but increases in pathologic pregnancy compared with non - pregnant controls. It is consistent with the study done by Kruse et al. (2008), who showed that significant lower levels of Th1 + type cytokines e.g IL-2 were observed that lower mRNA levels of the Th1-type cytokines (IL-2 and IFN-y) were observed during pregnancy compared with non -pregnancy female controls. Normal pregnancy is accompanied by decreased production of type 1 pro-inflammatory cytokines and increased production of type 2 anti-inflammatory cytokines [6,7]. IL-2 was decreased may be because it is a Th1 type pro -inflammatory cytokine. The decrease observed in Th 1 cytokines is associated with the presence of factors that inhibit the production of Th1 cytokines and these factors are important in the proliferation and differentiation of the trophoblastic cells and placentation and play a protective role on the fetal - placental unit as an attempt by the organism to maintain the pregnancy process (Feliciano et al., 2012). Despite this, Th1 cells have an essential role in the implantation and placental development. Hence, there exist a balance between Th1 and Th2, and this Th1/ Th2 dichtonomy aids in the explanation about the environment of cytokines underlying a successful pregnancy (Feliciano et al., 2012).
According to Robison and Klein, (2012), the depression of pro - inflammatory cytokines was associated with high HCG serum levels, of which the effect on cytokines production is not entirely clear. There may be other early pregnancy signals which may not only have a profound effect on immune regulation at the fetal maternal interphase but also influence the cytokine expression pattern within blood cells (Robison and Klein, 2012). IL - 2 was decreased from first trimester to the third, decreased more from first to second trimester than from second to third trimester to the third, trimester. This is consistent with Kruse et al., (2000), who was able to detect reduced IL - 2, IL - 18 and IFN - Y mRNA expression levels during the first trimester of normal pregnancy. Marzi et al., 1996 [9] showed that IL - 2 decreased in all trimesters compared with non- pregnant controls and that elevated IL - 2 serum concentrations have been found during the first trimesters in women who later develop pre-eclampsia. (Denney et al., 2011), indicated an overall decrease in pro-inflammatory cytokine trajectories in the innate and adaptive arms of the immune system and increase in counter- regulatory cytokines as pregnancy progresses. There was no significant difference between TNF - α levels in pregnancy and non-pregnancy controls. This supports the study of Jones et al. (2000). Kruse et al. (2000), showed that TNF - α mRNA levels were slightly higher compared with age - matched non- pregnant women. TNF - α was stable in all the trimesters. In the beginning of pregnancy, intense vascularization and cell proliferation helps the development of the embryo and the placentation, thus the presence of proinflammatory cytokines such as TNF - α is important at this early stage. It modulates trophoblastic growth and the trophoblastic invasion of the spiral arterioles. Although its presence is essential, overreaction can restrict the invasion and contribute to the pathophysiology of pre - eclampsia (Peracoli et al., 2007) [17]. Thus, it is essential to control its inflammatory response in the pregnancy. In other words, for TNF - α not to lead to pregnancy failure, it as to maintain a stable production profile in all stages. Hence, this is why level of IL - 10 remains high throughout pregnancy in order to regulate the level of TNF - α [18].
Conclusion
In this study the levels of four differ cytokines during physiologic pregnancy were determined. IL - 4 was significantly increased in pregnancy as compared to the non-pregnant state. Normal pregnancy is associated with changes in immunological parameters. This study showed a progressive increase in IL 24 from the first to the third trimester. Interleukin 10 being an anti -inflammatory cytokines or a Th2 -type cytokine was high in pregnancy compared with their non-pregnancy counterpart. IL - 2 was decreased from first trimester to the third, decreased more from first to second trimester than from second to third trimester to the third, trimester. There was no significant difference between TNF - α levels in pregnancy and nonpregnancy controls. In the beginning of pregnancy, intense vascularization and cell proliferation helps the development of the embryo and the placentation, thus the presence of pro- inflammatory cytokines such as TNF - α is important at this early stage.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!