Biography
Interests
Ray Marks
Department of Health and Behavior Studies, Teachers College, Columbia University, NY 10027, United States
*Correspondence to: Dr. Ray Marks, Department of Health and Behavior Studies, Teachers College, Columbia University, NY 10027, United States.
Copyright © 2021 Dr. Ray Marks. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Osteoarthritis, a chronic joint disease, and one possibly related to metabolic factors, and metabolic
syndrome, continues to be a highly prevalent painful disabling condition with few modes of
intervening successfully at any stage of the disease.
To examine what is known about the micro nutrient niacinamide and its various analogues and its
possible utility for intervening favorably to mitigate the progression of osteoarthritis.
The
Although not voluminous, the few pre clinical and clinical reports on this topic that prevail imply a
possible adjunctive role for niacinamide in the context of optimally activating selected biochemical
pathways that might in turn help to foster decrements in osteoarthritis pain and pathology as
experienced by many older adults.
Niacinamide and its analogues, which appear to have the potential to improve cellular metabolism
and energetics as well as reduce inflammation in aging cells may be useful for heightening the
outlook for many older osteoarthritis sufferers and should be studied further in the context of efforts
to minimize the immense burden of this progressive disease and to more ably enable clinically
relevant translation of several currently observed promising results.
Introduction
Osteoarthritis, a widespread painful disabling joint disease predominantly affecting older adults remains
extremely challenging to treat effectively, and safely. A disease strongly associated with inflammation, joint
swelling, joint deformity and varying degrees of bone pathology, as well as articular cartilage attrition,
physiological changes of the surrounding muscles and soft tissues of one or more commonly affected freely
moving joints that lead to muscle weakness, decreases in joint range of motion, and joint instability are
commonplace [1-3]. Arising from a widespread dysregulation of a group of enzymes termed the cytokines,
as well as various growth factors, prostaglandins, cartilage matrix fragments, neuropeptides, reactive oxygen
intermediates, proteolytic enzymes and protease inhibitors, alone or in combination these factors appear to
foster a progressive unrelenting cycle of cartilage bone, ligamentous and synovial tissue degeneration and
associated inflammatory responses that tend to sensitize both the peripheral and central nervous systems
[4]. As well, reactive symptoms of depression, oftentimes intractable pain, sleep problems, anxiety, fatigue,
a predilection for obesity and chronic diseases such as cardiovascular disease, and multiple functional
limitations, not only arise in due course and persist, but commonly limit social and economic participation
[5-7].
Although not well studied, and seldom recommended or discussed as a possible efficacious nutrient that might be helpful to examine and employ or ingest in the context of fostering promising safe and economic self-management strategies to counter the disease ramifications [8], the compounds known as niacin [formerly nicotinic acid]/niacinamide [formerly nicotinamide], and especially the latter has been shown to have an overall global and favorable antioxidant effect that can possibly protect against excess inflammation, as well as heighten osteoarthritis mobility, while decreasing osteoarthritis suffering [3,9-11]. A compound that is not only produced endogenously, to some degree, but one that can be safely administered orally, as capsules, tablets or liquids, as well as in food products, niacinamide, whose presence may well be objectively disturbed in the presence of osteoarthritis [12], in particular, has also been shown to have a favorable impact on cartilage tissues as well as on physical and mental health status. As well, an early report by a Dr. Kaufman, indicated there were objectively detected and verifiable arthritis pain and mobility improvements in response to the administration of mega doses of niacinamide when employed over an extended period. In addition, a controlled study of 445 patients supplied with niacinamide for 3-4 weeks with disabling osteoarthritis tended to yield observable improvements in joint range of motion and decreases in joint swelling in the treatment group that could not be attributed to a placebo effect even though all reported pain reductions [11]. A dose related response in favor of higher doses of the nutrient was also observed as well as a decrease in the subjects’ erythrocyte sedimentation rate and an overall 13 percent anti-inflammatory medication reduction.
However, even though osteoarthritis at that time, as well as at the present time has been deemed the most common form of joint disease, and one requiring multiple intervention approaches to offset its progression, almost no follow up of Dr. Kaufman’s 1940 observations, which were pursued with similar results over many years [13], were discussed or readily accepted in the medical context for decades [10,11,13]. Commonly assumed to be a progressive, degenerative disorder, the idea of any spontaneous arrest or reversal of the disease, which can potentially occur, was largely ignored, notwithstanding the observation of marked improvements in cases with rheumatoid arthritis, osteoarthritis, and those older adults reporting symptoms of stiffness but that were not yet diagnosed as arthritis, and that cessation of usage produced adverse outcomes or regression to former states of disability [13]. At the same time, conventional medications often effective for symptom relief, often caused significant side effects and did not slow the progression of the disease [14], while the role of biological pathways affected by precursors of certain nutrients such as NAD+ levels and nicotinamide [later renamed niacinamide] [15], and the development of various age associated pathologies, was largely ignored as well, even though niacinamide appeared safe and was found to improve inflammatory states and features of arthritis such as increased hand mobility, thus enabling more effective hand surgery in selected cases of hand arthritis [13]. One reason was that a role for mega doses of vitamins in the treatment or prevention of various forms of arthritis, although demonstrated to ameliorate selected arthritic conditions effectively with no untoward effects, was highly discounted in the medical field in its own right, as well as countered by emerging data demonstrating cortisone to be effective in treating various forms of arthritis, albeit with some risk [13].
Review Aims
In recognition of the role that might be played directly or indirectly by niacinamide or niacinamide analogues
in the presence of augmenting or replenishing any deficiencies or lowered rates of biosynthesis, intake,
and increased nicotinamide adenine dinucleotide [NAD+] usage [15], plus a persistent need to explore all
possible forms of therapy that might supplement or offer some improvements over current osteoarthritis
intervention approaches that are not always indicated or restorative, we elected to examine if any substantive
support or proof for this concept exists that supports an imperative to pursuing research on the niacinamide
molecule and its pathways in the context of comprehending the complexity of osteoarthritis pathology as
well as for mitigating any attribute or possible determinant of disabling osteoarthritis due to its involvement
in the NAD+ pathway.
Rationale
A wealth of data support the view that the biosynthetic pathway involving the coenzyme NAD+
[nicotinamide adenine dinucleotide] and that plays a critical role in the regulation of a host of key glycolitic,
metabolic and cellular functions is dependent on an adequate presence of niacinamide/nicotinamide. These
biosynthetic pathways that involve NAD+ and niacinamide, among others are not only of major import
in optimizing glucose and lipid levels, DNA repair, and stress responses, but also in regulating circadian
rhythms, chromatin modeling, energy metabolism, and transcriptional processes [15] that may have a bearing
on both the etiology and the pathogenesis of one or more degenerative diseases such as osteoarthritis, and its
prevention and amelioration, especially given its link to another effector pathway collectively known as the
sirtuins. Indeed, as outlined by Yang et al. [16] understanding that NAD+ homeostasis, which is vulnerable
to aging and disease processes, as well as niacinamide levels, has stimulated novel research efforts designed
to determine if replenishment or augmentation of cellular or tissue NAD+ [for example via niacinamide]
can have ameliorative effects on aging or age-associated disease phenotypes.
Methods
To examine the potential validity of the aforementioned rationale, and some possible support for the concept
of the merits of niacinamide as a possible beneficial direct or indirect arthritis moderator or mediator, a
comprehensive environmental scan and examination of this present topic of interest was undertaken using
the
After examining potential articles, those that were deemed salient were reviewed in detail and their main points extracted. It was felt that in view of the diverse nature of the data and its very limited focus on any one issue, only a very limited cursory descriptive report of some key observations was plausible.
The facts as presented here are thus possibly limited by one or more design flaws, and also possibly by the nature of the experimental models employed, as well as the limited numbers of robust focused clinical studies.
The literature is also very confusing at times, because it spans many years, as well as changes in nomenclature across time in some countries-not others-as well as discrepancies on what constitutes niacinamide versus biotin. Many diverse terms representing the current use of the term niacinamide and its analogues thus prevail, hence the terms applied in this report such as nicotinamide, niacinamide, and NAD+ (nicotinamide adenine dinucleotide) are those used and defined in the literature under discussion to represent one or more aspects of niacinamide and its biological associations as this might pertain to fostering a broader understanding of its influence on cellular processes such as those observed in osteoarthritis pathology and intervention possibilities and opportunities for treating osteoarthritis. Additional terms that appear in the literature and deemed synonymous with niacinamide for present purposes are: 3-pyridinecarboxamide; nicotinic acid amide; vitamin PP [Pellagra Prevention]; nicotinic amide; and vitamin B3.
Results
After a careful and dedicated electronic search for pertinent materials, only a limited number of articles
specifically focusing on niacinamide and osteoarthritis were found listed on
General Observations
Niacinamide, a critical enzyme found to occur naturally in several common foods, such as meats, and
reported to be highly relevant in the context of innate efforts to maintain cell health, appears to be involved
in several metabolic and protective processes as a component of vitamin B3, along with niacin. Although
challenging to link clearly as a possible upstream or downstream factor in the context of the complex but
well documented osteoarthritis disability cycle, among the small array of related articles that are cited in
most of the above data bases, several spoke to the possible role of niacinamide and/or its analogues as a key
biochemical precursor of the crucial NAD+ co-enzymatic redox and signaling pathways that suggests this
compound may indeed strongly influence many intracellular oxidation reduction processes that influence
aging processes including skeletal and neuronal tissue dysfunction [15,17] as well as key aspects of cellular
metabolism and cell fitness [15,18-20]. As such, even if not well studied in the clinical realm, it appears
niacinamide and its analogues are being interpreted as having considerable value worthy of future research in
the context of age-associated pathological conditions and others, and this idea cannot exclude osteoarthritis
pathology relevant linkages at this point in time. In particular intervening to reduce senescent cells associated
with damage, distress, and anti-inflammatory factors thought to possibly contribute to various disabling
conditions such as osteoarthritis and NAD+ dependent metabolic regulation processes that may well be
decreased in the progression of osteoarthritis appears to have some peripheral support, especially if one
considers the activity of a group of potential chondrocyte biological mediators collectively known as sirtuins
[15,21,22]. This is because sirtuins, such as sirtuin 1, as well as other members of the sirtuin family may
prove helpful in the context of osteoarthritic therapy development, especially those due to an associated
increased rate of age associated cell death [15]. As well, though not well studied, Deng et al. [23] noted that
sirtuin 1, a longevity gene as well as another sirtuin known as sirtuin 7-also NAD+ dependent- do appear
to be important regulators of cartilage homeostasis and development as well as bone [24,25].
As discussed by Braidy et al. [26], NAD+, an essential nucleotide present in all living cells and one strongly influenced by the presence of niacinamide serves as an important cofactor and substrate for a multitude of biological processes including energy production, DNA repair, gene expression, calcium-dependent secondary messenger signaling and immune-regulatory roles. However, its synthesis commonly declines with aging and age-related diseases, unless activated through an intrinsic pathway, or supplemented through the recycling of NAD+ from nicotinic acid, nicotinamide/niacinamide, and nicotinamide riboside [27]. In addition, according to Fredericks et al. [28], NAD+, an obligate co-factor for the catabolism of metabolic fuels in all cell types may become limited in availability in several tissues not only with age, but also during periods of genotoxic stress. Although the point at which NAD+ restriction imposes functional limitations on tissue physiology remains unknown, when examining this question in murine skeletal muscle, knockout mice exhibited a dramatic 85% decline in intramuscular NAD+ content, accompanied by fiber degeneration and progressive loss of both muscle strength and treadmill endurance, not unlike challenges one may observe in most knee osteoarthritis cases, and this occurred quite readily when one of the NAD+ pathways in the mouse model was blocked. In turn, administration of the NAD precursor nicotinamide riboside rapidly ameliorated functional deficits and restored muscle mass despite having only a modest effect on the intramuscular NAD+ pool. Additionally, the researchers noted that the lifelong over-expression of niacinamide phosphoribosyltransferase can preserve muscle NAD+ levels and exercise capacity in aged mice, supporting a possible critical role for tissue-autonomous NAD+ homeostasis and niacinamide analogues in the processes of efforts to maintain muscle mass and function [28,29].
In other research, Hamity et al. [30] implied efforts to increase and restore desirable NAD+ levels in damaged tissue environments and others, such as the application of niacinamide supplements, may yet protect against neuronal injury, and hypersensitivity to touch, possible important correlates of many painful chronic health conditions via nicotinamide riboside, a form of vitamin B3 and precursor of NAD+. The researchers suggested that agents that increase NAD+, a critical cofactor for mitochondrial oxidative phosphorylation systems and cellular redox systems, may hence represent a novel means of providing relief against certain peripheral neuropathies and their possible impact on pain and health status.
As observed in rodents, Elhassan et al. [31] found that even though NAD+ can be negatively modulated by conditions of metabolic stress as well as possibly by aging, it can also be positively impacted by applications of nicotinamide riboside. In an effort to establish whether oral nicotinamide riboside supplementation in aged participants would increase the skeletal muscle NAD+ metabolome and alter muscle mitochondrial bioenergetics, the researchers showed that the substrate did indeed elevate the muscle NAD+ metabolome. As well, muscle RNA sequencing revealed nicotinamide riboside mediated downregulation of energy metabolism and mitochondria pathways, without altering mitochondrial bioenergetics, while depressing the levels of circulating inflammatory cytokines.
Mitchell et al. [32] further noted that although the role of nicotinamide/naicinamide, the physiological precursor of NAD+ is somewhat elusive, it is possible to show chronic nicotinamide riboside supplementation not only improves health span measures, but can also augment depression of circulating inflammatory cytokines when applied to aged human muscle. According to Elhasson et al. [31] this process heightens the subsequent activity of the sirtuin family of NAD+-dependent deacetylases that help regulate apoptosis [cell death processes] in human chondrocytes [21], among other biological processes such as endocrine signaling, glucose homeostasis, aging, and longevity, plus the control of circadian clocks and mitochondrial biogenesis. This may be very important to examine further because sirtuin activity is often deficient in diseases such as type 2 diabetes, cancer, rheumatoid arthritis, cardiovascular and other age-relating diseases, and possibly osteoarthritis since they play an important role in the regulation of cell physiology, mitochondrial function, cell regeneration, and cell dynamics as far as preventing oxidative stresses in cells [33].
Research Observations
In accord with early reports by Kaufman [9], and a very convincing case study by Hoffer [13] a 1996 report
by Jonas et al. [3] described outcomes of a study that examined 72 cases with osteoarthritis randomized
for treatment with niacinamide or an identical placebo for 12 weeks. Outcome measures included global
arthritis impact and pain, joint range of motion and flexibility, erythrocyte sedimentation rate, complete
blood count, liver function tests, cholesterol, uric acid, and fasting blood sugar. Compliance was monitored
with a pill record sheet and interview. Results showed global arthritis impact improved by 29% in subjects
on niacinamide and worsened by 10% in placebo subjects (p=0.04). Pain levels did not change, but those
on niacinamide reduced their anti-inflammatory medications by 13%. Niacinamide reduced erythrocyte
sedimentation rate by 22% and increased joint mobility by 4.5 degrees over controls (p=0.04). Side effects
were mild, but higher in the niacinamide group (40% vs 27%, p=0.003).
Additional work by Sahin et al. [34] that aimed to examine the properties of oral supplementation of niacinamide and undenatured type II collagen on the inflammation and joint pain behavior in a rat model with osteoarthritis was able to show that osteoarthritis several associated harmful serum inflammatory compounds could be decreased significantly through the application of the niacinamide collagen compound.
In similar research designed to examine the potential solid-state interaction between ibuprofen and nicotinamide using thermal, spectroscopic, and microscopic techniques, it appeared that the solubility of ibuprofen, a non steroidal anti inflammatory agent, was enhanced by 62 times in the suspension when the concentration of nicotinamide was 13.3mg/mL. It was concluded by Oberoi et al. [35] that the suspension prepared in this way has the potential of being a more effective medication for pain relief in humans with osteoarthritis than ibuprofen alone.
In terms of efforts to understand why niacinamide might be an effective arthritic mediator or moderator, Hoffer [13] offered three possible explanations for reported favorable outcomes post ingestion of high dose naicinamide:
Niacinamide might provide a possible link to foster glycine metabolism.
Niacinamide might have important impacts on disease associated vascular systems.
Niacinmaide might provide a link that improves intra cellular oxidative processes.
Other research indicates niacinamide and other associated enzyme pathways may have the potential to suppress cytokine-mediated induction of nitric oxide synthase in various cells, while specifically influencing the signal transduction pathways that promote the synthesis and mediate the activity of interleukin 1, and/ or the NAD+ pool size [11].
Miesel et al. [36] who investigated whether the oxidative stress dependent activation of PARP or Poly (ADP ribose) polymerase, a family of proteins involved in a number of cellular processes such as DNA repair, genomic stability, and programmed cell death might play a role in the etiopathogenesis of arthritis showed daily doses of 4mmol/kg of niacinamide to suppress the measured prevailing arthritis indicators by 35%, while inhibiting the phagocytic generation of reactive oxygen species, which tended to increase six fold during the development of arthritis. This showed that consistent with Dr. Kaufman’s work the onset, progression, and remission of arthritis correlated positively to the ability of niacinamide to reduce oxidative stress induced alterations in cellular signal transduction pathways that are thought to play a pivotal role in the development of arthritis.
In other work, Xiong et al. [37] who examined the possible regulatory effects of niacinamide on intervertebral disc aggrecan in vitro found the glycosaminoglycan content in the nucleus pulposus was increased by 44.8% as compared to that of the control group. This group consequently concluded that niacinamide has the potential to up-regulate the expression of aggrecan in injured intervertebral discs, possibly through its ability to help protect the tissue from inflammatory interleukin beta-induced degenerative processes. Niacinamide was similarly found to help alleviate the effects of overload damage to the intervertebral disc and to foster recovery of the experimentally damaged intervertebral discs [38].
Indirect observations from a study on wound healing and fibroblast collagen production further showed an apparent increase in response following administration of a compound containing niacinamide [39] that may be pertinent in efforts to boost cartilage repair mechanisms. Moreover, nicotinamide in appropriate doses appears to protect against diabetes [11], a strong osteoarthritis correlate, as well as inflammation [10] as well as type II collagen arthritis when administered concurrently with thalidomide [40]. Niacinamide has also been found to be of considerable import as regards key DNA reparative and maintenance processes [15, 41], as well as of high significance in consideration of those inherent related sirtuin mechanisms designed to regulate chondrocyte energy metabolism and multiple chondroprotective processes [23, 24, 42].
The presence of niacinamide deemed to have the potential to modify a variety of toxic cellular processes [43], and possibly to slow or retard cell destruction and functional failure [10], as well as appearing to potentially promote cartilage matrix production through its ability to reduce the presence of harmful inflammatory mediators released by the diseased arthritic synovial tissue [2] is also found to inhibit excess bone formation, often a problem in osteoarthritis [43]. Moreover, it appears careful efforts to raise intracellular NAD+ levels where suboptimal through niacinamide supplementation may provide a promising therapeutic strategy for mitigating a variety of age-associated degenerative diseases and extending one’s lifespan in the context of disorders associated with the accumulation of chronic oxidative stress, inflammation, immune dysfunction, fatigue, and impaired mitochondrial function, such as metabolic disorders [29, 43-53].
Discussion
Although strategies to counter osteoarthritis progression and reduce pain have ensued for many decades,
and some research in the 1940s documented quite remarkable benefits in response to, high dose niacinamide
administration among various arthritis cases, often warranting surgery, this research was not translated
either at that time or at any subsequent time to the clinical realm or research realm, with few exceptions. Indeed, despite quite remarkable documented results that were replicated in other venues early on, the
original findings and others have largely been ignored, overlooked, or discounted as an intervention of
possible merit by many in the medical field, and consequently no further research appears to have been
actively conducted in this realm since 1996 to support the possible utility of niacinamide in the context of
understanding or intervening upon the commonly destructive osteoarthritis joint disease processes. This
situation which appears hard to completely justify, appears to prevail currently as well despite quite consistent
promising original clinical findings, clinical benefits observed in numerous other analogous or common
accompanying chronic disease conditions, aging and inflammatory biology research, and increasing evidence
that key NAD+ energy pathways and their homeostasis are clearly linked to the availability of adequate
niacinamide levels and could be impacted adversely by its deficiency. At the same time, almost no headway
has been made in the context of preventing, treating, or reversing any aspect of the complex osteoarthritis
process, and this remains a burgeoning highly disabling health condition with immense public health and
social implications in all aging societies and one where nutritionally based chondroprotective therapies is
highly sought [10].
In short, even though a sizeable number of clinical cases were found to apparently respond favorably to niacinamide when administered in high doses over prolonged periods, and cessation or a reduction of the therapy typically heightened the osteoarthritic joint damage that were otherwise seen to be averted with continued therapy [10], no recent work to replicate such potentially valuable findings could be located. As such, it is impossible to estimate how much undue suffering might have been incurred over time, given that vitamin B added to a diet may be all that is needed to provide some degree of arthritis relief and to engender a more favorable health impact than not [54]. Conversely, since low dietary vitamin B may foster joint space narrowing and osteophytosis in vulnerable women [55], as well as other health conditions involving the cardiovascular system [54], along with many metabolic challenges, and fatigue, it seems this topic is surely understudied and must warrant some consideration at the present time if one considers the number of opioid deaths alone that currently prevail due to unrelenting bouts of chronic osteoarthritis pain.
At the same time, accumulating evidence that certain oxidative processes that are potent destructive cartilage mediators can be impacted by niacinamide, and that an NAD+ age associated decrease produces a host of multiple common pathologies, but that many of these catabolic processes can be minimized through supplementation of its precursors, including niacinamide, more concerted research efforts to examine the niacinamide-NAD+ linkage in the context of osteoarthritis seems strongly warranted [19]. Such research may not only provide highly valuable insights as to why niacinamide may be of great import to the wellbeing of older adults with intractable forms of osteoarthritis, for example, those who are overweight or exhibit muscle weakness [51], but may solidify the current research on sirtuins as related to osteoarthritis and implied by Yang et al. [27]. For example, the impact of niacinamide on overall sirtuin based biology may explain its possible benefits when consistently administered, although its role in possibly reducing blood pressure and arteriol stiffness, comorbid chronic health conditions and clinical features, including cardiovascular and metabolic disease, inflammation, and anxiety [10] would undoubtedly have important highly relevant secondary impacts [19] as originally uncovered by Kaufman [9].
In sum, it appears more focused research to tease out the actual role niacinamide might play in the osteoarthritis disease cycle, and that includes the use of advanced technologies to explore cellular and molecular interactions between niacinamide and various forms and stages of arthritis, plus its role in NAD+ maintenance as this may offer valuable insights as regards various cognitive, vascular, metabolic, degenerative, and inflammatory processes implicated in osteoarthritis disability that might greatly advance current efforts to ameliorate the excess worldwide suffering attributable to an ever increasing osteoarthritis health burden. Moreover, since cost-effective osteoarthritis treatment solutions that are safe are highly desirable, it appears some of the health beneficial effects of diet and exercise recommended for osteoarthritis, which could derive in part from simply pursuing an optimally adequate niacinamide intake should be highlighted in future work [16]. Other possible benefits of targeted and tailored niacinamide usage in selected osteoarthritis cases may include overall health benefits, along with heightened self-efficacy for self-management practices, better sleep health, reduced helplessness and despondency, and a greater sense of control over their disease. Others that await assessment include reductions in depression, anxiety, and fatigue, as well as improvements in overall life quality [11].
In the interim, since mainstream and/or experimental drugs currently continue to fail to mitigate osteoarthritis suffering comprehensively and safely, and surgery is not always a viable option or indicated, research points to the possible utility of considering the continuous administration of adequate amounts of naicinamide as a natural product for supporting improvements and possible amelioration of those numerous osteoarthritis correlates that can possibly heighten the experience of intractable pain among those who are clearly niacinamide deficient.
Conclusion
The rationale for examining a component of vitamin B3 known as niacinamide as a key arthritic mediator,
and NAD+ attribute of high relevance appears to have substantive merit of high relevance to rheumatologists,
orthopedic scientists, surgeons, and clinical health promoters and practitioners as well as patients and
warrants concerted dedicated exploration, analysis and possible clinical translation of its possible physical
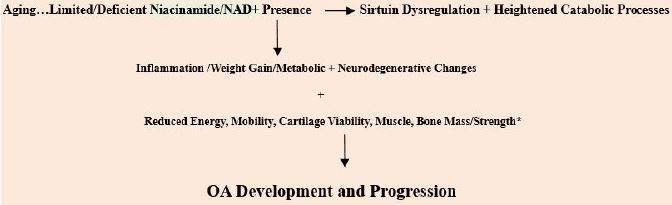
biological, anti-inflammatory, restorative, and psychological benefits as indicated thereafter. See Figure 1.
Bibliography