Biography
Interests
Ray Marks
Department of Health, Physical Education, Gerontological Studies and Services, School of Health Sciences and Professional Studies, City University of New York, York College, NY 11451, United States and Department of Health and Behavior Studies, Teachers College, Columbia University, NY 10027, United States
*Correspondence to: Dr. Ray Marks, Department of Health and Behavior Studies, Teachers College, Columbia University, Box 114, 525W, 120th Street, New York, NY 10027, United States.
Copyright © 2018 Dr. Ray Marks. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Osteoarthritis, the most prevalent musculoskeletal disease remains relatively impervious to successful treatment. Moreover, available treatments may not be indicated, or efficacious, and even if they are can prove toxic and commonly fail to impact the disease directly. Vitamin C or ascorbic acid, a well-established anti-oxidant with biological proven tissue based impacts, is generally considered to have little viable impact in the broad picture of this disease. We present some data on this topic that argue for more focus on the anabolic and catabolic potential of this compound in the context of osteoarthritis. Its role in symptom management is also discussed.
Introduction
Vitamin C, also termed ascorbic acid, is an extensively studied highly potent water-soluble vitamin found throughout the body that cannot be produced internally in humans [1,2]. A critical mediator of tissue biology, growth, development, and cofactor for several key enzymatic cartilage and bone reactions [3], vitamin C also exhibits well-established powerful anti-oxidative and anti-inflammatory properties [1,2,4]. Quite well examined in the context of several diseases where oxidative stress and its impact on cell viability is said to be implicated, such as neurodegenerative diseases [5], metabolic syndrome and obesity [6] - all important osteoarthritis correlates - vitamin C seems to be relatively understudied, albeit potentially of high import to the understanding of the etiology and clinical management of this complex multifactorial disease, as well as efforts to effectively engineer cartilage [7], the predominantly affected tissue.
Aims
Since oxidative stress is known to influence the outcome of arthritis adversely [8], and the alleviation of oxidative stress, among other goals, is said to be key to effectively intervening to moderate osteoarthritis disease and its progression [9], and to possibly improving life quality in a disease where very little progress has been made despite decades of research, this work aimed to examine if there is a sufficient basis for continuing to explore the possible role of vitamin C in the context of the osteoarthritis disease cycle.
Methods
To achieve this aim, a mini-review of available documents housed in PubMed and Scopus from 1980 up until May 16, 2018 using the key terms Vitamin C and Osteoarthritis, or Ascorbic Acid and Osteoarthritis-a chronic disease of unknown etiology affecting the whole synovial joint, and currently deemed to affect high proportions of older individuals across the globe irreversibly and very detrimentally was undertaken.
To this end, only salient research articles or reviews were selected, and their utility was verified through careful study of all potentially pertinent articles, including in vitro and in vivo studies. All forms of osteoarthritis were deemed acceptable, along with all modes of experimentation. No systematic review was possible at all, and it is acknowledged the body of data may not be exhaustive-but the two data bases employed were chosen because they presumably house state of the art and gold standard papers on this topic sufficient for arriving at a reasoned opinion.
Results
The data examined revealed only a small number of relevant studies, if compared to other themes in osteoarthritis research such as pharmaceutical drugs (eg 120 vs 1053). These were highly diverse and included various forms of molecular, cellular, pre-clinical and clinical research of various osteoarthritis joints and subpopulations. No unifying theme could be extracted from these data-however. Moreover, while some support for the possible salience of vitamin C in improving our understanding of the osteoarthritis disease process was noted, the data offered no clear cut convincing evidence either for or against either its anabolic or its catabolic influence or both in this regard, and many varying conclusions were evidenced.
However, of great apparent import in the context of osteoarthritis, which mainly affects older persons, is the finding that vitamin C is an important antioxidant and co-factor for numerous biochemical reactions [4] especially the synthesis and assembly of cartilage collagen [10-12], aggrecan and proteoglycans [11-14], and post-translational modifications of collagen [11]. Vitamin C also has important anti-inflammatory properties in addition to having the ability to influence cartilage matrix deposition by articular cartilage chondrocytes [15].
In this context, Blackburn et al. [13] who studied the expression of the sodium-dependent vitamin C transporter 2 system, the only known sodium coupled vitamin C transporter isoform present in articular cartilage, found that the expression of this transporter was significantly altered in human osteoarthritic tissues, which may suggest this is either an upstream causative factor or a downstream factor that can mediate or moderate chondrocyte cell biology and articular cartilage collagen composition. Stabler et al. [12] did indeed find this transport mechanism and chondrocyte vitamin C storage to be linked and in addition, that the presence of the inflammatory mediator TNF-α associated with osteoarthritis pathology inhibited intestinal ascorbic acid uptake, again implying a further possible pathogenetic link of vitamin C to this disease. In addition, data produced by Subramanian et al. [16] strongly suggested a role for vitamin C in both the synthetic as well as the degradative processes found in osteoarthritis, while Indranil et al. [17] found cases with knee osteoarthritis showed a significant decrease in vitamin C that correlated with radiographic changes. Conversely, in a study conducted by Plotnikoff et al. [18], the odds of having hip osteoarthritis were found to be 1.9 times lower in those individuals consuming recommended or higher than recommended levels of oral vitamin C (OR: 0.52; 95% CI: 0.29,0.96), and Surapaneni et al. [8] found a significant decrease in vitamin C levels among cases with osteoarthritis compared to controls.
Malicev et al. [19] however, found-vitamin C induced rather than prevented apoptosis in a cell culture of chondrocytes, which was dose dependent, even though McAlindon et al. [20] concluded that high intake of antioxidant micronutrients, especially vitamin C may reduce the risk of cartilage loss and disease progression in this group of adults. Nonetheless, Gallagher et al. [21] found no evidence to support or refute the role of vitamin C for purposes of chondroprotection, even though Muraki et al. [22] found vitamin C to display a possible protective role in women with knee osteoarthritis.
Earlier however, Kraus et al. [23] reported their group found all forms of vitamin C increased, rather than decreased the degree of oxidative damage in articular cartilage explants taken from guinea pig cartilage. Similarly, Sharma et al. [24], Li et al. [25] and Chaganti et al. [26] found higher serum vitamin C levels were more prevalent among those cases of knee osteoarthritis that exhibited more rapid rather than slower rates of disease progression. Kurz and Schulke [27] consequently cautioned against the use of high vitamin C doses in the context of treating arthritic diseases.
The use of vitamin C as an additive to therapeutic approaches to counter osteoarthritis continues to be examined however. For example, Hahn et al. [28] concluded that adding vitamin C may enable better outcomes in efforts to forge cartilage regenerative products, for example by adding it to injectable hydrogels as proposed by Rampichova et al. [29].
As well, cases prescribed intra-articular glucocorticoid injections have been shown to benefit more readily from these injections when these are combined with vitamin C, as have osteoarthritis cases being treated with hyaluronic acid [15]. This may be due to its anti-oxidative properties, as well as its collagen synthesis role, among others functions. Indeed, Chiu et al. [30] showed vitamin C decreased apoptotic processes and the expression of pro-inflammatory cartilage chondrocyte cytokines and matrix metalloproteases, even with low doses of vitamin C, a finding also noted by Ibold et al., [31].
Chang et al. [32] similarly found vitamin C not only protected human chondrocytes against damage from apoptosis, but that it also stimulated proteoglycan expression, as well as collagen formation, and its presence inhibited chondrocyte differentiation that usually occurs in the presence of oxidative stress, a finding supported by Kim et al., [33]. De Arruda et al. [34] too reported the uptake of a form of vitamin C known as L-ascorbic acid measurably improved the thickness of the calcified and non-calcified cartilage tissues in their model.
Moreover, exposure to vitamin C, results in telomere elongation and replicative lifespan increases of cultured chondrocytes [35], which may be helpful in osteoarthritic situations where patients appear more susceptible to oxidative damage than controls [36].
Sharma et al. [24] further showed vitamin C supplementation following a period of static loading reduced the normal degenerative processes of chondrocyte cells caused by static loading, while improving the cellular health and functioning of articular cartilage. Likewise, Koike et al. [9] found a vitamin C derivative to effectively suppress mitochondrial superoxide generation and to delay cartilage degeneration in a mechanical overload model.
Research by Wang et al. [37] had shown vitamin C may be especially beneficial for reducing bone size and numbers of bone marrow lesions, both important factors in the osteoarthritis disease cycle. Sufficient levels of vitamin C may also promote tendon, and muscle collagen synthesis, plus bone density, all possible structural changes that could lower the degree of any existing osteoarthritis pathology. Vitamin C also has the capacity to reduce joint injury risk [38], pain [39-44], inflammation [45-48], and muscle atrophy [49], to foster tendon growth and healing processes [50], and bone health [37,51]. Alone, or combined with selenium, vitamin C may hence be especially important in the prevention or therapy of mechanicallyinduced osteoarthritis as noted by Burger et al., [52].
DiscussionOsteoarthritis a debilitating disease with no known cure, is one where any form of palliative treatment that is non-toxic and encourages mobility would be highly prized. However, very little has been done to intervene in retarding or reversing this condition to date and the disease remains a very intractable problem with a worldwide growing prevalence. In addition, even where standard treatments are applied, many persons with this condition cannot take the recommended medications, or undergo surgery, and efforts to apply regenerative principles to diseased osteoarthritic joints while encouraging, remain suboptimal.
Substrates that can be identified early on as contributing towards the pathology and limited or eliminated as well as those that can be added safely to improve outcomes are thus important to examine, and may provide much in the way of improving the scope of understanding the disease, as well as its prevention and remediation. One substrate that has received very limited attention, but appears to have considerable potential for advancing our ability to treat this multifactorial disease and its complex etiology and treatment requirements is vitamin C, a well-established anti-oxidant with observed anti-inflammatory properties.
However, unlike pharmaceutical drug studies and studies on vitamin D and osteoarthritis, very limited data on this topic prevails, and that which is published is largely limited to discussions and experiments at the preclinical or cellular level, and a resultant body of highly heterogeneous conclusions of limited clinical utility.
Nonetheless, in consideration of a reasonable body of clinically relevant data, it does seem reasonable to conclude that more studies designed to examine the efficacy of vitamin C and the consequences of deficient as well as excess vitamin C plasma and synovial fluid levels, could prove highly beneficial.
In this regard, more clinically oriented well-designed randomized controlled trails, as well as comparative studies and case control studies might permit some form of meta-analyses to be conducted in the future and a better ability to tease out trends, and the implications of the diverse modes of delivering vitamin C in intervention studies, plus substrates and dosages examined, among other factors. Some consensus on the most reliable method of measuring vitamin C availability in the clinical realm and examining the status of the cell membrane and vitamin C transport mechanisms in patients with his disease can potentially help as well to isolate the importance of vitamin C in the osteoarthritis disease process.
In the interim, seems reasonable to accept that vitamin C is clearly essential for normal collagen synthesis, including collagen X [54,55], a major structural element of articular cartilage, and its surrounding tissues [56] and may hence be an important disease factor if its presence is suboptimal or excessive. As well, it is important to note, osteoarthritis patients may well be at high risk for vitamin C deficiencies that may exacerbate osteoarthritis pathologies as a result of their osteoarthritic disease state [7], poor diet, ageassociated declines in vitamin C levels, comorbid diseases caused by oxidative damage, and the presence of obesity [5,53], among other factors. These include, but are not limited to the presence of inflammation that often accompanies osteoarthritis [16], and vitamin C transporter deficiencies in the muscles, tendons and bone structures surrounding the osteoarthritic joint.
Vitamin C also helps to regulate the energy status of maturing chondrocytes, plus related alkaline phosphatase activity [34], and in addition to its powerful antioxidant properties [2], can foster wound healing processes and others such as the synthesis of several osteoblast-related proteins [35]. On the other hand, wellknown consequences of a vitamin C deficiency include fatigue, joint swelling, muscle aches and pains, bone alignment and emotional changes [37].
Although no distinct cause and effect relationship can be deduced between osteoarthritis pathology and vitamin C deficiencies, the presence of a vitamin C deficiency can undoubtedly prove debilitating, as well as accelerate rather than decelerate the prevailing rate of joint destruction and its spread to other initially unaffected joints as depicted in Figure 1. The finding that excess dietary fat and cholesterol-associated with obesity-a recognized osteoarthritis determinant may possibly exacerbate or promote a chronic vitamin C deficiency [57] is an especially intriguing finding in the context of osteoarthritis-where obesity is highly common.
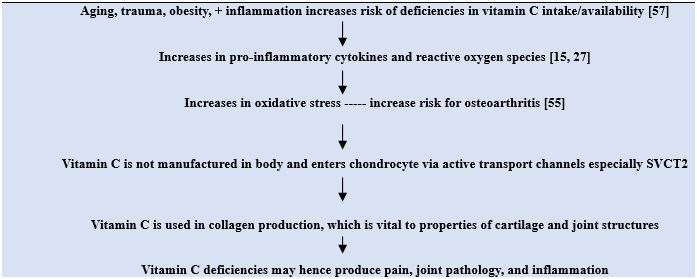
On the other hand, the thoughtful administration of vitamin C where this is clearly deficient may help to alleviate pain, and would be of specific import given the detrimental side-effects of opioids and other pain relieving remedies that may have to be used to control osteoarthritis pain. Indeed, alone, or coupled with appropriate doses of targeted mechanical stimulation, more evidence than not implies the presence of optimal levels of plasma, serum, and joint vitamin C may yet assist in improving the life quality of the osteoarthritis sufferer quite immensely, and its role in fostering the mechanical properties of regenerating articular cartilage remains highly promising [58].
So what can a clinician do in the interim? According to Kurz et al. [53] they might consider examining their clients for any possible vitamin C deficiency, or risk for a deficiency. In addition to encouraging intake of fruits and vegetables that are excellent sources of vitamin C, helping those who have limited income to obtain these foods if this is problematic, as well as considering the impact of alternate forms of vitamin C delivery may be helpful [31]. The risk of excess vitamin C intake should also be a treatment-based consideration. Those at high risk who are likely to be the elderly, those with comorbid cardiovascular conditions, those with inflamed joints, those with limited income, and the obese patient should be especially targeted, along with those taking anti-inflammatory drugs, those on restricted diets, and those with a cancer history [1].
The researcher in the interim might make highly valuable contributions in this field in all these realms and especially by employing validated outcome measures of joint function, biomechanical test procedures, functional outcomes, and research designs that can tease out or examine various osteoarthritis subgroupings and states of prevailing clinical osteoarthritis. To strengthen conclusions of clinical studies in an area where little recognition of the ‘whole’ joint being affected in this disease and its biomechanical and loading properties in any study context is visible, more longitudinal radiographic and biopsy studies may be helpful. Testing whether administering vitamin C is able to readily enter the chondrocyte, for example through nanotechnological approaches or injection of a safe vitamin-C permeable derivative [29,31,59], and examining the possibility of the vitamin C transporter mechanism as a therapeutic target [60] may prove especially beneficial in efforts to ameliorate this cycle of abnormal extrinsic and intrinsic sites of pathology, especially perhaps in the early on in the disease process, rather than later phases [61]. In addition, efforts to examine whether combining vitamin C and vitamin E [27], along with the co-administration of vitamin C and conventional drugs [62,63] appears worthy of further exploration [63,64]. The role of laserfacilitated vitamin C delivery [65], and the use of L-ascorbic acid and other vitamin C analogues to repair articular cartilage defects should also be explored further [66].
Conclusion
Based on the available evidence, and in agreement with Hart et al. [67] and French and Clegg [68], it seems reasonable to conclude that osteoarthritis pathology and its development may clearly be influenced in a number of ways by prevailing vitamin C levels known to modulate or mediate articular cartilage matrix biosynthesis and degradation processes, as well as immune function, pain, and inflammation, plus bone integrity and structure [3]. A well-known scavenger of free radicals and reactive oxygen species [69], it also seems feasible to conclude a low level of vitamin C will prevent or impede the repair or maintenance of collagen, which may be one root cause of osteoarthritis pathology. By contrast, the clinical well-being of the osteoarthritis patient may be markedly heightened by careful vitamin C assessments and recommendations, the co-administration of other supplements and drugs, and the lifespan of cartilage explants may be significantly extended by the addition of vitamin C to the constructed tissue. In short, further topical studies are not only highly warranted to advance the field but may foster new insights and advances in the modes of preventing, treating and managing this prevalent debilitating and highly disabling arthritic disease [70].
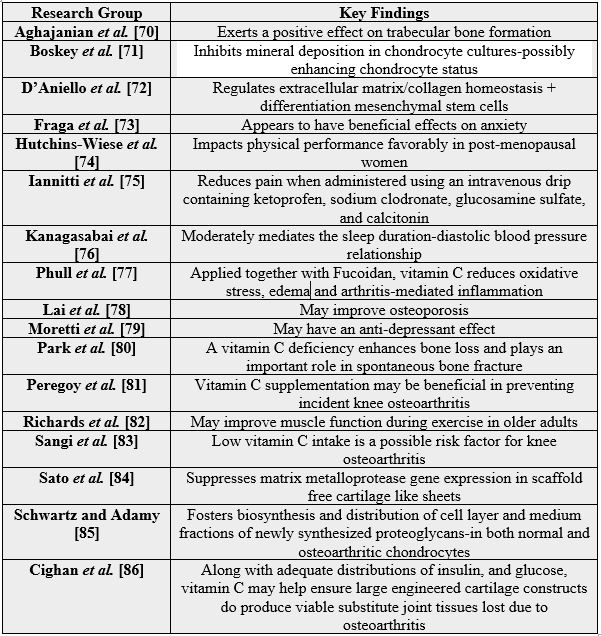
Indeed, given the many ways vitamin C can interact with joint tissues [see Table 1], vitamin C supplementation - where warranted - may not only yield beneficial symptomatic effects, for example on pain [40], but indirect effects on joint health, in general, though its influence collagen and other related joint structures, as well as associated health conditions, such as osteoporosis and hip fractures [71] and the inflammatory aspects of osteoarthritis [72]. Those with high degrees of joint pathology [73] may benefit most significantly, hence, efforts to examine the role of vitamin C in preserving or fostering joint integrity and further verification of the results shown below are highly desirable [74].
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!