Biography
Interests
Maria Cardoso, J.1*, Alba Pérez2, María Casas, A.2 & Marta Armengod2
1Department of Psychology and Sociology, University of Zaragoza, Zaragoza, Spain
2AFEDAZ: Association of families with Alzheimer Disease
*Correspondence to: Dr. Maria Cardoso, J., Department of Psychology and Sociology, University of Zaragoza, Zaragoza, Spain.
Copyright © 2019 Dr. Maria Cardoso, J., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The ability to understand and attribute mental states, including intentions, knowledge and desires,
to both ourselves and other people, is referred to as possession of a ‘Theory of Mind’ (ToM).
Some studies have revealed an association between ToM abilities and Executive Functioning (EF)
capacities Although many types of research had documented executive dysfunction in Alzheimer
Disease (AD), the link between executive functions (EFs) and ToM is not yet fully studied in AD.
Therefore the main aim of the present study is to know if patients with early AD are able to resolve
a First Order False belief task.
A total of 22 patients with early Alzheimer´s disease were recruited into the study from a dementia
speciality clinic in Zaragoza, Spain. In this study, we tested a first-order belief task. Executive
function was assessed using “Executive functions and frontal lobes test” (BANFE-2).
Patients with AD showed mild and moderate scores in the BANFE-2. When we analysed the data
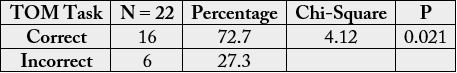
we found that 72,7% of the subject can resolve the task (χ² = 4.12; p = 0.021). When we analysed
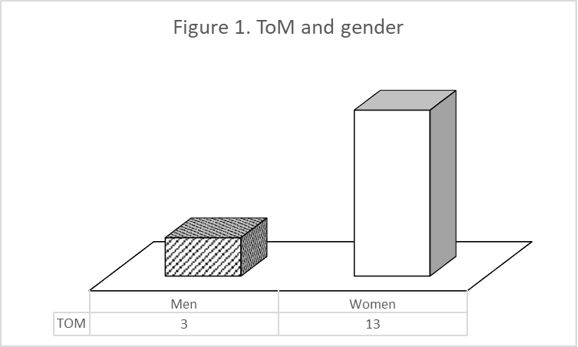
the data according to gender we found that women resolve the TOM task better than men (χ² =
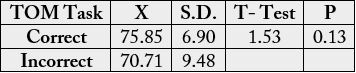
6.26; p = 0.012). There were no age differences in TOM task.
The data of this research showed that patients with AD were able to attribute first-order False
Beliefs. We hypothesized that compensatory mechanisms could allow functional social interaction
in AD patients.
Introduction
The ability to understand and attribute mental states, including intentions, knowledge and desires, to both
ourselves and other people, is referred to as possession of a ‘Theory of Mind’ [1,2]. These ‘mentalizing’
abilities form an essential and fundamental role in many social and communicative interactions, allowing
successful and mutual exchanges of information between individuals [3,4]. The mechanisms underlying
Theory of Mind (ToM) abilities are not as yet clear. Evidence has supported the notion of a modular
structure underlying ToM abilities, with separate component parts involved in specific, differing mentalizing
processes [5,6].
False-belief tasks are one of the tests most often used to assess ToM abilities in both typically and atypically developed individuals [7]. False-belief tasks involve scenarios in which individuals are shown a situation where reality states differ from belief states, and where a clear distinction between one’s own current belief states and the current belief states of another individual is created. One of the first false-belief tasks designed to assess self and other belief-attribution abilities was Perner, Leekham, and Wimmer’s ‘Smarties’ task [8]. In this task, children were shown a box of sweets (‘Smarties’) and asked what they thought would be inside.
On responding to sweets/chocolate, children were shown that the box actually contained pencils. The pencils were then re-hidden, and children were asked three critical questions, akin to the following: ‘What did you think was in the box before you saw inside?’ (self-oriented belief attribution); ‘What would your teacher, who hasn’t seen inside, think was in the box?’ (other-oriented belief attribution); and ‘What was really in the box?’ (reality test).
Some studies have revealed an association between ToM abilities and Executive Functioning (EF) capacities [9,10]. In typically developed adults, it has been demonstrated that a heavier cognitive load can influence success on ToM tasks, often resulting in more egocentric errors, supporting the notion of a relationship between ToM and EF [11,12]. Keysar, Barr, Balin, and Brauner [13] reported a study in which two adults (a participant and a confederate, the ‘director’) sat on opposite sides of a table, with a vertical grid between them. Some of the slots on the grid were occluded so that only the participant, but not the director, could see them, creating distinct self and other-person perspectives. The task required participants to follow the director’s instructions in reassigning positions to various objects. Some objects were unique (e.g. a truck) and others were ambiguous, such as three candles (small, medium, large). In the case of the candles, the smallest candle, from the participant’s view, may have been occluded so that, if the director said ‘move the small candle’, he would be referring to the medium sized candle, which is the smallest from his perspective. Using eye-tracking equipment, these authors found that participants were sometimes biased by an egocentric interpretation of instructions - even when they clearly knew that information was shared or privileged, they still focused on the occluded objects as potential solutions. These findings have been replicated across different studies, suggesting a resilient effect of egocentric biases in adults in implicit tests of ToM [14]. These results indicate that even in typically developed adults, who arguably should possess the cognitive mechanisms necessary for successful ToM expression, EF abilities (e.g. inhibition, attention direction) may not automatically be recruited when ToM is assessed implicitly, and may only be utilized when explicitly required. Some contradictory evidence about the relationship between ToM and EF has also been reported. Fine, Lumsden and Blair [15] report a case study of patient B.M., who suffered from amygdala damage and had been diagnosed with schizophrenia and Asperger’s syndrome. Patient B.M. was shown to be severely impaired in his ability to represent mental states (ToM) but did not show any impairment in his EF abilities. However, in the majority of cases, studies continue to find a link between EF and ToM abilities, both within clinical populations [16] and in non-clinical populations [17]. People with Alzheimer Disease (AD) are unable to resolve complex ToM tasks with a high demand for reasoning skills and executive functions [18].
Although many researches had documented executive dysfunction in Alzheimer Disease (AD) [19], the link between executive functions (EFs) and ToM is not yet fully studied in AD. Therefore the main goal of the present study is test if patients with early AD are able to resolve a First Order False belief task.
Materials and Methods
A total of 22 patients with early Alzheimer´s disease were recruit into the study from a dementia specialty
clinic in Zaragoza, Spain. Inclusion criteria included having been diagnosed with dementia and having
a poor performance in executive function. Patient diagnosis was derived by multidisciplinary team who
performed extensive behavioral, neuropsychological and neuroimaging assessments. Demographic data are
presenting in table 1. Subjects received no compensation for participating in the study.
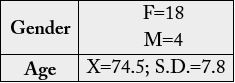
In this study, we tested a first-order belief task. A piggy bank with coins inside it is presented and we asked to the the experimental subject “What do you think is in the piggy bank?” Subsequently, with the collaboration
of the subject with dementia, we replace the coins with marbles. Finally, we ask subject what would someone
else believe that is inside the piggy bank.
Executive function was assessed using “Executive functions and frontal lobes test” (BANFE-The BANFE-2
consists of fifteen tasks used and validated in neuropsychological settings for assessing executive functions
mediated primarily by the frontal lobes. This test helps clinicians conduct an extensive and accurate
assessment of damage and functional impairment of the frontal lobes. Tests include Sorting Test, Verbal
Fluency, Color Word Interference Test, Tower Test, Mazes, Gambling Test, Metamemory Test, Category
Generation, Viso-Spatial Working memory (two different tasks), Verbal Working Memory (three tasks).
This test has been developed and standardized for Spanish-speaking populations. We used BANFE-2 to
evaluate executive functioning.
Compliance with the standards contained in the Declaration of Helsinki on human experimentation was
guaranteed at all the times. Inclusion criteria included having been diagnosed with dementia. Exclusion
criteria included presenting visual disease or another disease that could affect the carry out of the task.
Participants were tested in a single session, lasting between 5 and 10min in order to avoid fatigue. Patients
completed the task individually in a room with no noise or other stimuli that could affect the performance
in the task. The task was conducted always by the same experimentalist to avoid individual differences in the
experimental team.
Data was analysed with SPSS 20.0. The statistical techniques were descriptive (arithmetic mean and standard
deviation) and group comparative (student´s t test). We used Chi Square test for non-parametric tests.
Differences were deemed significant when α < .05.
Results
Patients with AD showed mild and moderate scores in the BANFE-2 (X= 80.1; S.D. = 6.53).
When we analysed the data we found that 72,7% of the subject can resolve the task (Table 2).
When we analysed the data according to gender we found that women resolve the TOM task better than men (χ² = 6.26; p = 0.012) (Figure 1).
There were no age differences in TOM task (t = 1.53; p = .13) (Table 3).
Discussion
Social cognition encompasses various explicit and implicit psychological processes that permit individuals to
gain benefits from being part of a social group [20]. These processes, which occur through social interactions,
may be elicited by verbal or non-verbal stimuli. Social stimuli, such as facial expressions, body postures,
prosody, hand gestures, or eye gaze directions, give important signals about the surrounding environment.
They may, for example, alert for the presence of danger or for the location of a particular area of interest to
investigate. These external cues help people to learn about the world through others’ experiences without
undergoing the trials and errors themselves. Showing emotions, noticing and understanding others’ emotions,
and responding to them with empathy and sympathy are all prosocial behavioral processes. These may be
automatic and unconscious, but all help individuals of a group feel closer, enhance collaboration, and inhibit
aggressive interactions. ToM, an important concept of social cognition that is also known as “mentalizing,”
refers to the ability to attribute mental states such as beliefs, desires, and knowledge to oneself and others, as well as to appreciate that these may differ from our own1. ToM is often divided into “affective ToM” and
“cognitive ToM,” referring to the ability to mentalize others’ emotional and cognitive states, respectively.
Closely related to ToM, meta-cognition is the ability to reflect on our own actions and thoughts and to
notice alterations in our cognitive abilities [21].
Decety and Jackson [22] examine converging lines of evidence from lesion and functional neuroimaging studies suggesting empathy derives from three main cognitive processes. In the first step, the other’s emotion is ‘shared’, activating brain areas involved in subjective emotional experiences such as the inferior frontal cortex, superior temporal cortex, amygdala, right somatosensory cortex, right temporal pole and right insula [23]. Second, one recognizes that the initiating agent of this subjective emotional experience is the other, not oneself. This ability to determine that the source of an internally represented emotion or intended goal is located outside of oneself requires perspective-taking (PT), which appears to be mediated by a brain circuit including the medial prefrontal cortex [24]. This process also requires the capacity to assign agency, which is likely mediated by the heteromodal association area at the junction of the temporal, parietal and occipital lobes [25]. Finally, the ability to accurately infer the other’s perspective requires the intentional suppression of one’s own viewpoint [26]. Both functional and developmental lesion studies in humans suggest that the frontal pole, approximately corresponding to Brodmann’s area (BA) 10, may perform this regulatory function, actively inhibiting the selfperspective in order to allow the other’s perspective to be considered [27].
Many neuropsychiatric disorders are associated with deficits in empathy, including schizophrenia, Asperger’s syndrome, sociopathy, post-traumatic brain injury and stroke. A group of diseases that is of particular interest to the question of how brain damage leads to loss of empathy are neurodegenerative conditions that occur after normal social development has been established. Loss of empathy is an early and central symptom of frontotemporal lobar degeneration (FTLD), a focal neurodegenerative disorder involving primarily the frontal and temporal lobes. FTLD patients with predominantly temporal damage show dramatically increased interpersonal coldness [28] and have pathologically low levels of cognitive and emotional empathy, while FTLD patients with primarily frontal atrophy seem to lose the capacity for empathic PT, but do not become significantly colder as a group. Alzheimer’s patients, on the other hand, do not typically show significant changes in empathy [29].
Although significantly less frequent than in frontotemporal dementia patients, AD patients often present with behavioral changes at onset, such as disinhibition and social awkwardness [30,31]. Disinhibition, social awkwardness, and apathy have been reported as the first symptoms in 6.9%, 5.0%, and 2.0%, respectively, of patients with AD [30]. Social inappropriateness seen in AD is probably multifactorial, in part secondary to altered social cognitive abilities such as perception of social cues, impaired processing, and loss of control over reactions. Time of onset of these deficits concurrent with other cognitive symptoms during the course of the disease is still unclear.
The main goal of this study was to discuss the implication of ToM in early AD. Our results are congruent with the studies cited above. The data of this research showed that patients with AD were able to attribute first-order False Beliefs. In a work carried out by Gregory [32] the only task on which Alzheimer´s disease group displayed deficits was the second false belief task. This task places very heavy demand for working and episodic memory.
Our finding confirms that cognitive ToM abilities decrease progressively over the course of AD. ToM reasoning relies on both cognitive resources (executive process) and mentalizing ability. The executive functions in our patient group are moderate affected, so these subjects could develop compensatory mechanisms to resolve the task. Cognitive ToM reasoning in early stages of AD continues to be efficient because of the alteration use nonspecific executive resources.
Also, we found differences relative to gender. We found that women resolve the TOM task better than men. This fact is in agreement with other studies suggesting that females are portrayed as more nurturing and empathetic, while males are portrayed as less emotional. Some authors suggest that observed gender differences might be largely due to cultural expectations about gender roles [33].
Conclusions
The decreased in ToM ability and executive functioning in AD patients at different stages of the disease allow
us to consider EF as a scaffold to a successful ToM reasoning. As reported by Stern [34], we hypothesized
that compensatory mechanisms could allow functional social interaction in AD patients.
In conclusion, the current study provides empirical evidence of a progressive decay of cognitive ToM abilities in AD. This finding needs further empirical confirmations in order to understand behavioral changes in real interpersonal interactions.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The present study was supported by a research grant from Departamento de Innovación, Investigación y
Universidad. [S57_17R EDUCAVIVA - Educación y Procesos Psicológicos].
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!