Biography
Interests
Tadesse Teferra, F.
School of Nutrition Food Science and Technology, College of Agriculture, Hawassa University
*Correspondence to: Dr. Tadesse Teferra, F., School of Nutrition Food Science and Technology, College of Agriculture, Hawassa University.
Copyright © 2019 Dr. Tadesse Teferra, F. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This review summarizes the nature, types and properties of inulin polysaccharides and their applications as prebiotic dietary fibers. Natural food and commercial plant sources of inulin and extraction methods are presented. The physico-chemical and functional properties of inulin are summarized. The prebiotic roles of inulin and their mechanisms of action are detailed. Inulin acts as prebiotic dietary fiber with multiple putative health benefits. It reduces caloric intake and contributes to reduced blood glucose and plasma lipid/cholesterol levels when used as sugar and fat replacers. It also stimulates immune systems and protects the colon mucosa against carcinogenesis and inflammation. Inulin also alters the composition and population of the gut microbiota. It stimulates the growth and activities of health beneficial microorganisms while inhibiting enteropathogenic bacteria. The beneficial microorganisms ferment inulin and produces acids including short chain fatty acids that lower the pH in the colon and inhibit pathogens. The health beneficial bacteria also produce other metabolites that positively influence human health. The consumption of inulin is however, associated to symptoms of gastrointestinal discomfort, when consumed at higher levels to meet the daily recommendation of dietary fiber. Potential solutions to the limitations are forwarded as future research ideas and policy inputs.
Abbreviations
CVD = cardiovascular disease; DP = degree of polymerization; FOS = fructooligosaccharide; GI = Gastro intestine; HP = high performance [inulin]; ISAPP = International Scientific Association for Probiotics and Prebiotics; LPS = lipopolysaccharide; MDP = Mean degree of polymerization; OF = oligofructose; RDA = recommended daily allowance; SCFA = short chain fatty acids.
Introduction
Inulin is a naturally occurring polysaccharide as a storage carbohydrate in tens of thousands of plants. Unlike
starch, which is the most abundant storage polymer of glucose, inulin has mainly fructose as monomeric units
and a glucose endpoint. Inulin is found in more than 30,000 plants and the main commercial sources are
tubers of Jerusalem artichoke (Helianthus tuberosus) and dahlia (Dahlia pinnata) as well as roots of chicory
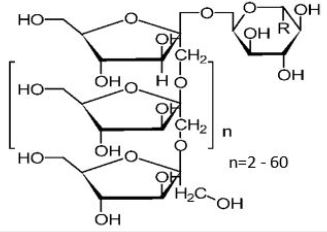
(Cichorium intybus) and yacon (Polymnia sonchifolia) [1,2], [Figure 1 (A), (B), (C) and (D), respectively].
In plants, inulin naturally exists as a mixture of oligo- and polysaccharides of fructose ranging from 2 to 100
units depending on plant species, age and extraction techniques [3].

Inulin was discovered by Valentine Rose (German Scientist) in the 1800s as a plant carbohydrate from the
roots of Inula helenium and named in 1817 [1]. Rose found this peculiar polysaccharide as a water-soluble
extract and was able to isolate it from plant sources using boiling water. Later in 1864, a German plant
physiologist and a pioneer researcher in fructans, Julius Sachs, showed the spherocrystalline structure of
inulin from different plant roots. Inulin has a long history of being used as a sweetener for diabetic patients
[6].
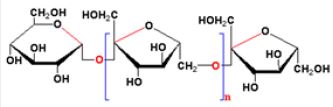
Inulin belongs to the fructan carbohydrate group (fructose based polymers) and is composed mainly of β-Dfructosyl
subgroups linked by (2→1) glycosidic bonds and the molecule usually ends with a (1↔2) bonded
α-D-glucosyl group [7] (Figure 2). The length of the fructose chains varies and usually ranges for inulin
between 2 and 60 monomers, but can reach 100. The fructans are often represented by the general formula
GFn, where G represents the units of glucose, F represents fructose units, and n refers to the number of
fructose linked together to form the entire carbohydrate chain [8].
Inulin with the glucose end have no aldehyde or ketone (carbonyl group) and hence does not have any reactive or reducing terminal, which makes it fairly stable. However, inulin usually represents mixtures of different forms of fructose polymers and it is inevitable that fructans with no glucose ends exist including mono and di saccharides, which can take part in many reactions including the Maillard type browning [9]. Inulin is completely hydrolyzed into the monomeric units at acidic pH (<3) and high temperature (90- 100℃) in a matter of 30 to 40 minutes [10]. In the pH ranges relevant to food application, however, there was no degradation at a temperature as high as 100℃ for extended time (55 min) [11], which suggests that inulin can be used as ingredient in many food systems and still remain stable under severe processing conditions.
The physico-chemical and functional properties of inulin are linked to degree of polymerization (DP) as well as the presence of branching in the structure. The short-chain inulin fraction, [oligofructose (OF), DP <10], is much more soluble and sweeter than native and long-chain inulin, and can contribute to improved mouth-feel as its properties are closely related to those of other sugars [1]. The degree of sweetness of low DP inulin groups is comparable to fructose. Inulin with higher DP are used as a fiber-type prebiotic with numerous putative health promoting effects [12].
The crystalline structure of inulin has a 5-fold helical structure and can be produced in two differently shaped morphologies: obloid and needle-like, by varying the cooling temperature of inulin from solutions [9]. The needle like crystals increase viscosity, while the obloid ones improve lubricity (mouthfeel) of foods.
Similar to many other polymers, the solubility of inulin is dependent on DP. The solubility of inulin decreases with increase in DP. Solubility was also reported to increase with temperature [13]. Wada, Sugatani, et al. (2005) [13] also demonstrated that enzymatically synthesized inulin (from sucrose) with higher DP (16-18 units) had higher solubility than native counterpart.
Inulin exists in different favored conformations in solutions depending on various factors. Helical conformation was reported to be favored for lower DP inulin (DP ≤5) [14,15]. The stability of zigzag and many other conformations were also reported by Vereyken, Van Kuik, et al. (2003) [16], implying the flexibility of inulin as ingredient in foods where the favored conformational arrangement of the ingredients play roles in stability or specific properties of the system.
The term ‘inulin-type fructan’ refers to all β- (2←1) linked linear fructose polymers (Figure 2) including
native inulin (DP 2-60, MDP = 12), oligofructose (OF) (DP 2-8, MDP = 4), and inulin HP [high performance,
(DP 10-60, MDP = 25)] as well as Synergy 1, a specific combination of OF and inulin HP [17]. The
different subcategories of inulin-type fructans, varying in their DP, are appealing for different applications
in the food industry. Detail of the application of inulin in the food industry will be given in this review.
The other major group of fructose polymers are called ‘levan-type frucatns’, which are characterized by their β-(2-6)-linked fructose residues (Figure 3). Levans are also called phleins in plants and are found in abundance in the grass families [18]. They play closely similar roles as inulin type fructans, although they have not been as extensively studied. Dahech, Belghith, et al. (2011) [19] showed the anti-diabetic, hypocholesterolemic and anti-tumorigenic activities of levans in animal models. Other studies reported that levan-type fructans can be used as food ingredients for different functional purposes such as color and fragrance carrier as well as fat replacers [20-24]. For the interest of this review, however, discussions are limited to the inulin groups as prebiotic agents in human health.
The levels of inulin in human diets vary depending on geography and diet differences. The western diet is
reported to provide a range of values (1-10g/day), where the range for the US population was indicated
to be lower (between 1.3 and 3.5) compared to European diet (3 to 11g/day) [25]. Commonly consumed
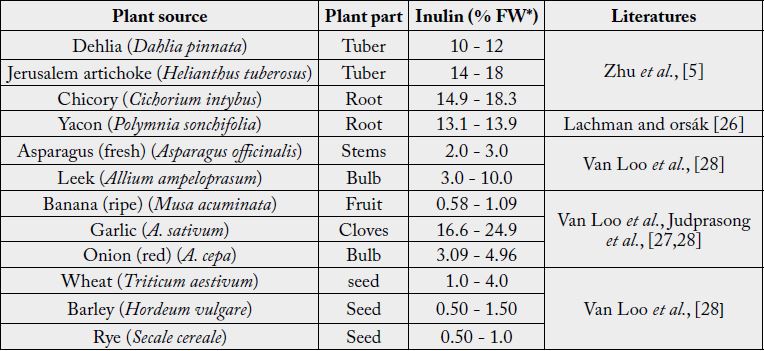
plant-based foods such as onion, leek, garlic, banana, wheat, rye and barley, provide appreciable quantities of
inulin in human diets [9]. The major plant sources, tubers of dahlia and Jerusalem artichoke, as well as roots
of chicory and yacon (Figure 1), have fairly higher contents [5,26] (Table 1), for they use inulin as energy
reserve and are used for commercial extractions. Many commonly consumed vegetables and fruits as well as
cereals have varying levels and types of inulin. From the data summarized in Table 1, it can be noted that, if
consumed frequently in adequate amounts fresh fruits like banana [27,28] and vegetables like asparagus and
leek [28], as well as cereals like wheat barley and rye [28], can greatly contribute to the daily dietary fiber and
prebiotic requirements for humans. Spice-vegetables like garlic and onion are also important contributors,
although, their amount included in foods is limited.
*FW = fresh weight
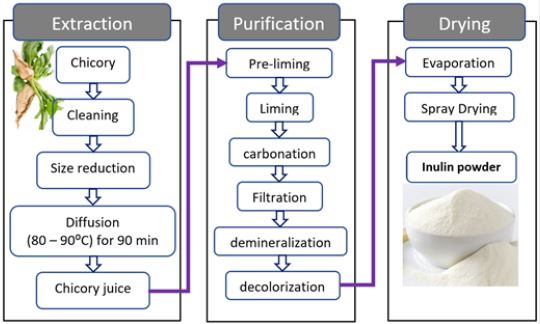
The conventional extraction of inulin is done almost exclusively from chicory roots due to its suitability and desired DP of the extract [5]. The production of inulin is achieved in three major steps extraction, purification and drying (Figure 4). The extraction process begins with cleaning and size reduction operations. After size reduction (slicing or pulping) diffusion of the granules will follow, which is achieved by heating to 80 - 90℃ for 90 minutes. The purification process involves liming, carbonation, filtration, demineralization and decolorization. The purified slurry is then gently evaporated and finally spray-dried.
Inulin is used as ingredient in foods for many purposes. The main applications are for texture modification,
prebiotic action, sugar replacement and/or bulking agent for intense sweeteners, fat replacement and dietary
fiber roles [6,29]. The low molecular weight inulin such as fructo-oligosaccharides (FOS) or OF (DP 2 - 8)
are known to have excellent solubility and possess as good sweetness as glucose or 35 to 55% as sweet as sucrose
[6]. These properties make the lower molecular weight inulin, ideal sugar replacers for diabetic patients
or bulking agents for intense sweeteners.
The higher molecular weight inulin, specifically the inulin HP (PD >10), are known for their limited solubility and no taste (no sweetness). They impart smooth mouthfeel and this makes them important fat replacers [6,29]. Inulin HP cut caloric intake and serve as prebiotic and dietary fiber when used in foods and are believed to greatly contribute to improved human health. Their importance as prebiotics for direct actions on immune stimulation, anti-tumorigenic property and improvement in nutrient (minerals) absorption as well as their indirect action via alteration of the gut microbiota has gained greater attention over the last decades (more on this later).
Prebiotic Role of Inulin
The term prebiotics has been defined for the first time in 1995 by Gibson and Roberfroid (1995) [30]. There
have however, been inconsistencies in the definitions of prebiotics over the years. The basic concept that
prebiotics being indigestible components of foods that selectively stimulates the growth and activities of
health beneficial groups of gut microbiota such as Bifidobacterium and Lactobacillus among others [6,30-33],
remained the back bone of all definitions used in literatures. In August 2017, experts’ consensus statement
on the definition of prebiotics was release by the International Scientific Association for Probiotics and
Prebiotics (ISAPP); and now a prebiotic is defined as “a substrate that is selectively utilized by host microorganisms
conferring a health benefit” [34].
Prebiotics are characterized to have three defining criteria [35]: (i) indigestibility - resistance to gastric acidity, enzymes and absorption; (ii) fermentation by health promoting intestinal microflora and (iii) selective stimulation of the growth and activities of groups of bacteria associated with improved health and wellbeing of humans or animals. Inulin-type fructans are resistant to digestion by human enzymes and are selectively fermented in the colon by health beneficial microbes. They therefore, increase the diversity and population of the health promoting bacteria, while inhibiting pathogenic groups [2,17].
Inulin were also reported to have antioxidant activities and exert protective action against oxidative damages to digestive organs. Pasqualetti, Altomare, et al. (2014) [36] demonstrated that inulin’s protective action against lipopolysaccharide (LPS)-induced oxidative damage to the human colon mucosa regardless of cooking and digestion treatments, showing greater stability, which adds to their prebiotic properties.
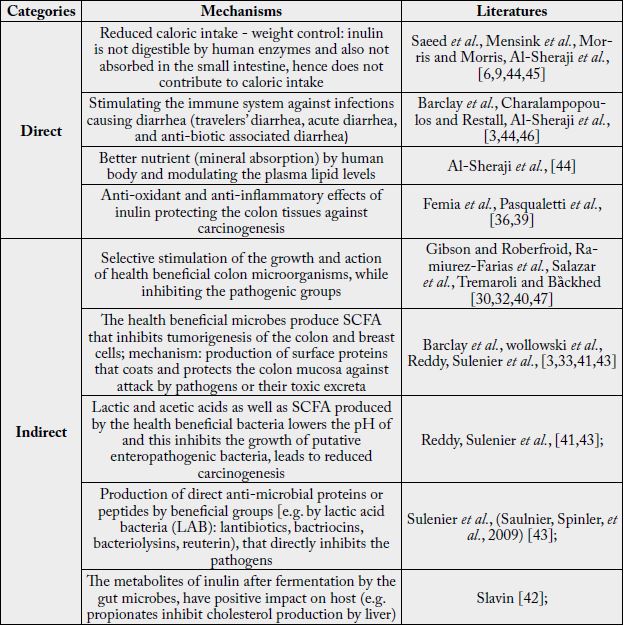
The mechanisms by which inulin and other prebiotics benefit host has been studied for over three decades
now and is still on going. For the purpose of this review, the mechanisms of inulin action on host health is
classified into direct and indirect types. The direct actions are those immediate effects of the consumption of
the prebiotic inulin on the health of the host. One of the ways inulin plays health beneficial roles on humans’
body is by reducing caloric intake when used as fat and sugar replacers [37,38]. Inulin is also known for its
anti-oxidant activity [36] and anti-inflammatory (protective) effects [39]. Inulin also play roles in reducing
plasma lipid and cholesterol levels. All of these are relatively immediate and direct impacts the consumption
of inulin is associated to.
The effect of inulin on the population and diversity of colon bacteria is considered slow and indirect action, which is by far the most studied area. The general understanding so far, is that inulin and other prebiotic dietary fibers selectively stimulates the growth of the health beneficial groups of colon microorganisms in the expense of the harmful ones. The major health beneficial bacterial groups (with examples) of great interest to human health includes [30,40]:
• Lactobacilli (Lactobacillus acidophilus, L. casei, L. delbruekii),
• Bifidobacteria (Bifidobacterium bifidum, B. adolescentis, B. longum, B. infantis), and
• Streptococci (Streptococcus salivarius sub-species thermophilus, S. lactis).
The consumption of inulin was reported to alter the proportion of the different groups of bacteria in human colon (Figure 5). Supplementation of prebiotics (fructo-oligosaccharides) was shown to increase the proportion of bifidobacteria by 3.67 times and decreased the percentage of harmful bacteria (bacteroides, clostridia and frusobacteria) by 5 to 10 times.
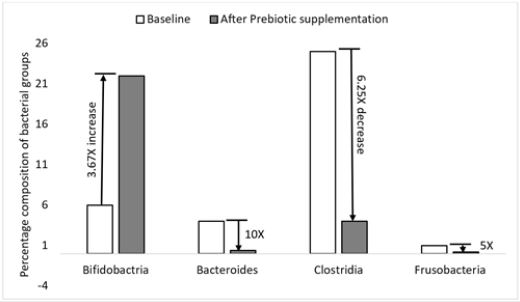
The mechanisms by which health beneficial bacterial groups such as Bifidobacterium and Lactobacilli potentially promote the health and wellbeing of the host are many and the major ones are summarized in Table 2. It is believed that the microorganisms produce acids including short chain fatty acids (SCFA), that lower the pH of the colon, which inhibits the growth and activities of the harmful bacterial groups [41]. Butyrate is preferentially used by the colonocytes as energy source, which helps them fight inflammation and carcinogenesis. Similarly, propionates are also known to interfere and inhibit cholesterol production by liver of the host [42], which helps cut the plasma cholesterol level and reduces chances of cardiovascular disease (CVD) complications. The health beneficial groups of microbes have also been shown to produce different peptides and proteins that are directly toxic to the pathogenic groups. Sulenier et al., [43] reported that lactic acid bacterial groups produce components such as lantibiotics (class I), bactriocins (class II), bacteriolysins (class III), that directly inhibit the growth of enteropathogens including Escherichia coli. It was also indicated that L. reuterii secretes reuterin, another effective toxicant against the pathogenic groups. This is evident that the health beneficial microbes may produce many more toxic substances that inhibit growth of the pathogenic groups.
Basically, inulin is safe for consumption and there is no reported toxicity either in animals or humans.
However, ingestion of inulin at higher levels (20 - 30g/day to attain the recommended daily dietary
fiber recommendations), was reported to cause gastro-intestinal (GI) intolerance [48]. Studies also showed
that increased flatulence and bloating were experienced by human subjects in different studies. In a study conducted to see the effect of inulin on the blood lipid level [49], daily intake of 14g inulin caused various
GI symptoms and could not result in targeted hypolipidemia. In a similar study, 18g/day inulin incorporated
in to low fat diet was fed to human subjects and was reported to cause increased flatulence, abdominal
cramping and bloating compared to a control diet (without inulin) [50]. Similar observations were documented
in studies involving supplementation of inulin levels of 10g/day or more. Other studies indicated
that consumption of 10 -15g/day of inulin was tolerable in humans [25,51], with great individual variations.
For optimal health benefits, it seems evident that as much native inulin as can be tolerated by individuals together with frequent consumption of adequate levels of foods rich in inulin may be advisable. Systematic delivery of the tolerable levels of inulin as supplementation or fortification should be practiced with recommendation of frequent consumption of foods rich in inulin. Promoting frequent consumption of the good inulin sources such as garlic, onions, asparagus, leeks, bananas, artichokes, and chicory root should be integral part of the health and nutrition plan for communities with low dietary fiber intake like the US population. Research may be required if combining the supplementation and/or fortification of the 15g/day and having the dietary sources can help avoid the intolerance. Frequent consumption of other soluble dietary fibers of similar functions on human health with no or minimal GI discomforts need to be researched.
Another possibility of having optimal health benefits, that may need to be investigated, is the possibility of in vitro fermentation of inulin by cocktail of the health beneficial groups of bacteria and supplying them (entire broth: metabolites and bacterial cells) with different foods. This requires intensive investigation in terms of suitable carrier foods, safety and the ability of those target organisms and their metabolites passing through the extreme conditions of the upper GI sections with minimal degradation. Propagation of the microbial cells using inulin as substrate and administering them (probiotic form) in encapsulated forms may also be looked into as an alternative delivery mechanism.
Summary and Future Research Focuses
Inulin is a naturally occurring polysaccharide derived from plants which makes it a safe and label friendly
food ingredient. The process of extracting and purifying inulin from natural sources is apparently easy, clean
and also cheap. Inulin was found to be very stable to various extreme conditions (temperature and pH) and
enzyme actions that gives it versatility for use in wider range of food, nutraceutical and pharmaceutical
products.
The use of inulin as prebiotic fiber and its associated benefits have been documented (previous sections of this review). The drawbacks and proposed resolutions are briefly. Due to its resistance to digestive enzymes, stability to low stomach and high intestinal pH, as well as due to its exclusion from absorption in the lower GI, Inulin consumption, directly contributes to lower caloric intake and blood glucose level. Consumption of inulin is associated to other positive health benefits. Inulin is also protective against carcinogenesis of the colon and breast cells. It was also well established that inulin has immune stimulating effect and its consumption is associated to better resistance to different forms of diarrhea. Inulin consumption is also related to improved mineral absorption.
The main and probably the most important prebiotic action of inulin is its ability to selectively stimulate the growth of health beneficial bacteria in the expense of pathogenic groups, which is considered indirect impact in this review. This means that inulin completely alters the diversity and population of gut microbiota within few weeks of consumption (5g/serving, 3 times a day). The health beneficial bacteria ferments inulin and converts it into lactic and acetic acids. Production of SCFA as metabolites is also common. Extensive production of the acids results in inhibition of the pathogens by lowering the colon pH. The health beneficial bacterial groups (bifidobacteria and lactobacilli) also produce peptides and proteins that have inhibitory effects on the pathogens.
High stability to extremes of conditions and physical properties such as sweetness of low DP, makes inulin a good sugar replacer or a competitive bulking agent for high intensity sweeteners. The smooth mouthfeel imparted to food matrices also makes inulin a natural and healthy alternative additive as fat replacer. Moreover, high DP inulin such as inulin HP categories, do not have flavor and their addition to foods does not affect the sensorial taste desirability. Inulin solubility depends on the DP and can be used as additive (as functional or nutraceutical) in foods to a desired consistency.
The major limitation of inulin application in foods, causes GI symptoms including flatulence, bloating, nausea, abdominal cramping and other complications to various degree in humans at higher doses. This limits the prebiotic application of inulin to only 10 - 15g/day, which is half way below the recommended daily allowance (RDA) for dietary fibers. However, it can be assumed that, if 100g of the other food sources (vegetables, fruits, cereals and spices) (Table 1) can be consumed per day (separately or in combination) the remaining 50% can easily be attained. This could lead to realization of the RDA levels of dietary fiber by the community. But this may still cause the GI discomfort and the possible remedies will be discussed in the next subsection.
It is known that inulin is important for human health as a prebiotic dietary fiber. There are however, many
aspects of its optimal applications for human benefits, that are yet to be understood. Future research should
look into ways of modifying inulin delivery to minimize the intolerance symptoms experienced by consumers
at higher doses. The influence of DP on the GI discomfort also needs to thoroughly be investigated.
Other methods of reducing the intolerance symptoms and increasing inulin dose to meet the RDA of
dietary fiber should be looked into. For instance, coating the inulin or attaching it with some proteins that
modulates the interaction of inulin with the receptors in the upper and middle GI may help reducing the
discomfort and allow administration of increased doses.
Alternative ways of utilizing inulin that need to be studied may include in vitro fermentation by the health beneficial bacterial groups and administering the entire fermentation broth (microbial cells and metabolites) in to the human GI. This and other ways of optimizing the benefits of inulin as prebiotic is of great interest to avoid the GI discomfort. However, extensive researches in developing systems that meet the safety standards and testing them in animal and human subjects are required and with greater priority. Alternatively, the characteristics of the in vitro fermentation system need to be researched for compatibility with food systems including fermented dairy products (yoghurt, cheese) and others like pickles and sausages.
Researchers and professional communities may need to lobby for policy level commitments for funding. Inulin type prebiotic dietary fibers need to be seriously taken into considerations in planning interventions. Mandatory fortification of the tolerable level inulin in to staple foods and strong recommendation and promotion of the consumption of inulin rich sources are crucial for rapid improvement of community health in countries with regular diets far below RDA for fiber.
Conclusive Summary
The prebiotic roles of inulin and its modes of action is summarized. The known sources and the different
classes as well as the various applications of inulin is covered. The actions of inulin on human health as a
prebiotic (immediate/direct) and mediator of gut microbiota (long term effect/indirect) are suggested for
the first time. The possible ways of increasing its health impacts are also forwarded as future research directions
and priorities.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!