Biography
Interests
Alexandru Ciocarlan1, Aculina Aricu1 & Elisabeta-Irina Geană2*
1Laboratory Chemistry of Natural and Physiologically Active Compounds, Institute of Chemistry, Chisinau,
Republic of Moldova
2ICSI Analytics Group, National R&D Institute for Cryogenics and Isotopic Technologies (ICIT), Ramnicu Valcea,
Romania
*Correspondence to: Dr. Elisabeta-Irina Geană, ICSI Analytics Group, National R&D Institute for Cryogenics and Isotopic Technologies (ICIT), Ramnicu Valcea, Romania.
Copyright © 2019 Dr. Elisabeta-Irina Geană, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Plant resources is the main supplier of bioactive phytochemical compounds and has been for many years the major source of pharmaceutical, aromatic and industrial compounds. The therapeutic use of plant resources is essential to maintaining the health, well-being and functionality of the human body, a special role being for medicinal and aromatic plants, and wild fruits. The modern trend aims to use the natural products, both in prophylaxis and as adjuvants, in order to increase the therapeutic arsenal and to provide the body with familiar and easily assimilated products.
Here we report the results of RP-HPLC quantification of highly biological active triterpenes ursolic and oleanolic acids in extracts of four medicinal plants species, including: Common Marigold (Calendula officinalis L.), two Mentha sp.: Bergamot mint (M. x piperita var. citrata (Ehrh.) Briq.) and Ginger mint (M. x gracilis ‘Variegata’), Romanian Tufted Thyme (Thymus comosus Heuff. ex Griseb.), and six wild fruit species such as Cornelian cherry (Cornus mas L.), Dog rose (Rosa canina L.), Blackberry (Rubus fruticosus L.), Blackthorn (Prunus spinosa L.), Hawthorn (Grataegus monogyna Jack.) and Russian olive (Elaeagnus angustifolia L.). The samples are originating from Republic of Moldova and were harvested during the 2013 and 2014 seasons.
This study showed that the contents of OA and UA varied considerably with the plant material. Species with a high OA level were Cornelian cherry and Thyme, while UA was quantified in important amounts in Bergamot mint, Hawthorn and Thyme, and high content for Cornelian cherry. Statistical tools (Hierarchical Clustering analysis (HCA) and Principal Component Analysis (PCA)) applied to the analytical data allowed the classification of the investigated natural sources, highlighting the plant materials with high potential of triterpene bioactive compounds. These results can provide a starting point for the comprehensive utilization of medicinal plants and wild fruits, including also the potential resulted by-products for further superior valorization.
Introduction
The popularity of herbal medicines is connected with their ease access, therapeutic efficacy, low toxic effects
and relatively low cost [1]. The use of herbal medicinal products and supplements has increased tremendously
over the past decades with about 80% of the world population relying on them for some part of primary
healthcare [2,3]. Nowadays, the utilization of medicinal plants in pharmaceutical industry is being extended
annually by the introduction of new plant species in the new formula of medical preparations [4,5].
Medicinal plants and wild berries are distinguished by a complex chemical composition and triterpenes play a special role among metabolites. Pentacyclic triterpenes are mainly widespread in cuticular waxes which cover surfaces of different plant’s organs such as stem, bark or leaf and fruit [6].
The studies of oleanolic and ursolic acids occurrence in medicinal plants [7] and wild berries and their contribution on pharmacological properties are constantly reported [8,9]. Oleanolic and ursolic acid have a similar structure and are widely spread in plants worldwide. Both compounds show wide range of biological activities such as: antibacterial [10], antifungal [11], anti-inflammatory [12], anti-proliferative [13], antileukemic [14], diuretic [15], antidiabetogenic [16], hepatoprotective [17], cytotoxic [18], anti-HIV [19] and apoptotic effects [20], combined with low toxicity [4,5]
Rapid and accurate quantification of these metabolites is very important for assessing the quality of pharmacological materials. Different methods for extraction of active ingredients from plant materials, such as conventional extraction methods (maceration extraction, reflux and Soxhlet extraction, ultrasonic assisted extraction) [21] and green extraction methods (microwave-assisted extraction (MAE)) [22] have been reported. The reported methods for triterpenic acids analysis are capillary electrophoresis, high performance liquid chromatography with UV, ELSD or MS detection and gas chromatography [23].
The aim of this paper was the quantification of highly biological active triterpene ursolic and oleanolic acids (UA and OA) in four medicinal plants species, including: Common Marigold (Calendula officinalis L.), Bergamot mint (Mentha piperita var. citrata (Ehrh.) Briq.), Ginger mint (Mentha x gracilis ‘Variegata’), Thyme (Thymus comosus Heuff. ex Griseb.) and six wild fruit species such as Cornelian cherry (Cornus mas L.), Dog rose (Rosa canina L.), Blackberry (Rubus fruticosus L.), Blackthorn (Prunus spinosa L.), Hawthorn (Grataegus monogyna Jack.) and Russian olive (Elaeagnus angustifolia L.) in order to identify rich natural sources for a new group of phytotherapeutic preparations, medical devices, value-added products and new semi-synthetic active substances from raw materials of natural origin. All investigated species are well known due to their biological and pharmacological properties and are often used by population for treatment of various diseases [24]. The samples are originating from Republic of Moldova and were harvested during 2013 and 2014 seasons. The investigated wild fruits species collected form spontaneous flora are frequently used by local population in fresh, frozen or dried forms or processed by the herbal tea producers. The Russian olive (Elaeagnus angustifolia L.) is less commonly used by humans, but it is a preferred food source of birds, rodents and herbivores. The medicinal plants are frequently used in tea herbal composition, traditional medicine and some of them culinary, as condiments. The species Bergamot mint (M. x piperita var. citrata (Ehrh.) Briq.) and Ginger mint (M. x gracilis ‘Variegata’), Romanian Tufted Thyme (Thymus comosus Heuff. ex Griseb.) originated from the right side of the river Prut and recently were introduced in Republic of Moldova. The novelty lies in the fact that these species have not been studied so far in this regard.
Materials and Methods
The aerial parts of overage medicinal plants were collected at the full flowering stage during 2013 from
the Experimental subdivision of the Collection of Medicinal Plants (CMP) of National Botanical Garden
(Institute), Republic of Moldova, geographically located at N 46º58’ 25”, E 28º52’ 47”. The plants were dried
at room temperature (20-23ºC) in dry, dark and well-ventilated room. Voucher specimens of each cultivar
were deposited at the Herbarium of the National Botanical Garden. The plant material was mechanically
shredded in fragments about 5-6 mm before extraction. The wild berries were collected during 2014 season,
Ungheni district, Petresti village, Republic of Moldova, geographically located at N 47º18’ 25”, E 27º44’ 51”.
The extractions were made in such a way to be selective. At the first step, the raw material was degreased
to make easily HPLC analysis. The samples of medicinal plants (10g) were extracted using Soxhlet type
extractors in triplicate, ten extraction cycles each. First plants were degreased with light petroleum ether to
remove vegetable waxes, volatile oils and fats (Petroleic fractions, PF). Then triterpenic fractions (UA and
OA) were extracted with ethanol (Ethanolic fractions, EF).
The whole fresh berries (10g) were consecutively defatted by immersion in hot light petroleum ether (Petroleic fractions, PF), for five minutes, then extracted by immersion in hot chloroform (Chloroformic fractions, CF), for five minutes, in order to extract OA and UA from the berries cuticular layer.
The extracts were evaporated to dryness at 35ºC under reduced pressure using a rotary evaporator. Aliquots of each crude extract from medicinal plants (EF) and berries (CF) were dissolved in methanol using ultrasonication and filtered through a 0.45μm micro-filter before HPLC analysis.
Ursolic and oleanolic acids were quantified by means of high performance liquid chromatography (HPLC)
coupled with Photodiode Array Detector (PDA) using a previously reported procedure [25]. Analysis was
performed using a Thermo Finnigan Surveyor Plus HPLC System (Thermo Fisher Scientific Inc., San Jose,
USA) equipped with a Surveyor Photodiode Array Detector (PDA), Surveyor autosampler, Surveyor LC
Pump (quaternary gradient), and Chrome Quest Chromatography Workstation software. The OA and UA
from extracts were identified by their retention time and spectral data by comparison with standards. To
confirm peak identity among possible interference peaks in the vicinity, the technique of standard addition
to the sample was approached. Also, the purity for the interest peaks was satisfactory.
The OA and UA standards were purchased from Sigma-Aldrich (St. Louis, MO). Methanol and phosphoric
acid (98% p.a.) were HPLC grade and obtained from Merck. Twice demineralized water produced by a
Milli-Q Millipore system (Bedford, MA, USA) was used for the preparation of the aqueous solutions. All
the other reagents used in the present study were of analytical grade.
The obtained analytical data were processed statistically by analysis of variance (ANOVA) and used to
evaluate significant differences among different natural sources based on triterpenic OA and UA acids. The
Duncan`s test was used to discriminate the different medicinal plants and wild fruits species (p≤0.05). Principal
Component Analysis (PCA) and Hierarchical Clustering analysis (HCA) were performed in order to
discriminate between different categories of plant materials. All the mathematical and statistical analyses
were performed using Microsoft Excel 2010 and XLSTAT Add in soft version 15.5.03.3707.
Results
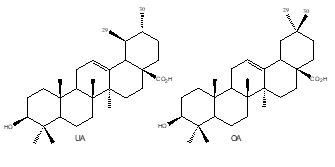
Oleanolic acid (OA) and ursolic acid (UA) are isomers and differ by positions of C-29 and C-30 methyl
groups (Figure 1). OA and UA always exist in the same plant, are chromatographically inseparable on normal
phase, therefore for their analysis the RP-HPLC is more appropriate [26].
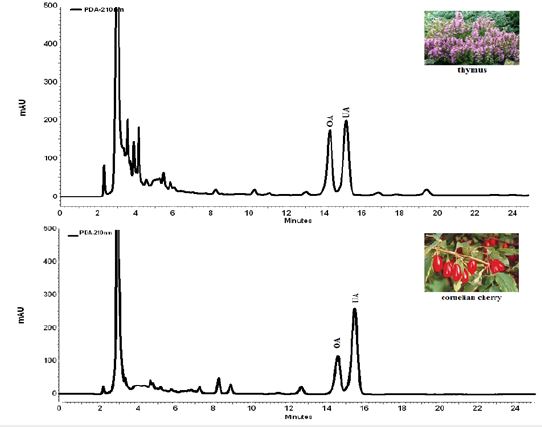
The HPLC system allowed successful separation and simultaneous identification and quantification of OA and UA in the plants and wild fruits extracts (Figure 2). The chromatographic peaks were identified by comparing their retention time and the UV spectra with that of each standard compound, which was eluted in parallel with the optimized mobile phases. In addition, spiking samples with the reference compounds showed no additional peaks, which further confirmed the identities of the peaks. Typical HPLC chromatograms of the extracts from two analyzed species were shown in Figure 2.
Quantification of OA and UA in Medicinal Plants and Wild Fruits Extracts
Generally, the UA and OA content from plant materials are expressed as mg/100g of dry plant or dry/fresh
fruit material (mg/100g dry weight, d.w.). In our study, the content of OA and UA was expressed as mg
OA, respectively UA in the obtained extracts and those extracts with high content of OA and UA bioactive
compounds being purified and then used to obtain new semi-synthetic active substances from raw materials
of natural origin. Also, the extract yield from plant material was estimated. Analytical performances of the
applied method of analysis were reported previously [25]. The experimental results are presented in Table 1.
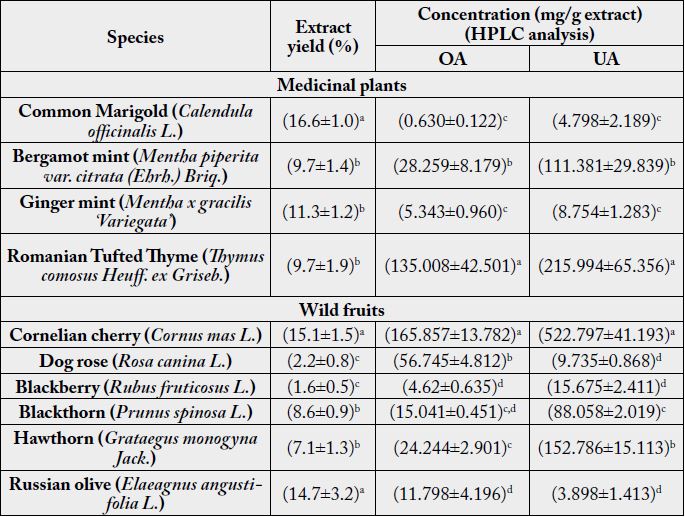
The values represent the mean of the results obtained for the three replicates ± standard deviation. Means with different lowercase letters in the column differ significantly according to the Duncans multiple range test at p≤ 0.05
The extract yield ranged from 9.7-16.6% for medicinal plants and from 1.6-15.1% for wild fruits, with higher values for Common Marigold, Cornelian cherry and Russian olive fruits. A high extract yield, does not involve a high content of OA and UA, the natural sources containing also other bioactive triterpenic phytochemicals like uvaol, sterols, hydroxy derivatives of UA and OA and their esters, phenolic compounds, etc.
OA content in the analyzed extracts varied from 0.630 - 135.008mg/g extract in the case of medicinal plants and from 4.62 - 165.857mg/g extract in the case of wild fruits (expressed as mg/g fresh fruit). Thyme and Cornelian cherry extracts contain high amounts of OA, while Common Marigold, Ginger mint and Blackberry contain lower amounts.
UA content in the analyzed extracts varied from 4.798 - 215.994mg/g extract in the case of medicinal plants and from 3.898 - 522.797mg/g extract in the case of wild fruits. Cornelian cherry, Hawthorn, Thyme and Bergamot mint extracts contain high amounts of OA, while Russian olive fruits, Dog rose, Common Marigold and Ginger mint contain lower amounts.
Thyme and Bergamot mint extracts together with Cornelian cherry, Hawthorn and Blackthorn extracts contain important amounts of OA and UA acids and could be considered as valuable natural sources of bioactive OA and UA phytochemicals. The availability of UA and OA from Tymus was estimated to be 1827.28mg/100g d.w. for UA and 1142.95mg/100g d.w. for OA, while Bergamot mint contain 1092.00mg/100g d.w. UA and 278.05mg/100g d.w. OA. Also, the triterpenic content of wild fruits (expressed as mg/g fresh fruit) was estimated for to be 78.94mg/g for UA and 25.04mg/g for UA in the case of Cornelian cherry, 6.47mg/g UA and 10.25mg/g OA for Hawthorn and 7.56mg/g UA and 1.29mg/g OA for Blackthorn.
Our data are in agreement with reported literature regarding the quantification of some triterpene compounds in various natural sources, such as Thymus sp. (aerial part) [27], Calendula officinalis flowers and Rosmarinus officinalis [28] and fruits [8,29]. It should be mentioned that authors express in different ways the amounts of analysed compounds.
The use of statistical analysis in food chemistry studies has increased because the results are easy to interpret
and discuss [30] and tends to be used to interpret the data in phytochemical studies. In order to evaluate
the variation in different natural sources, hierarchical cluster analysis (HCA) was performed based on the
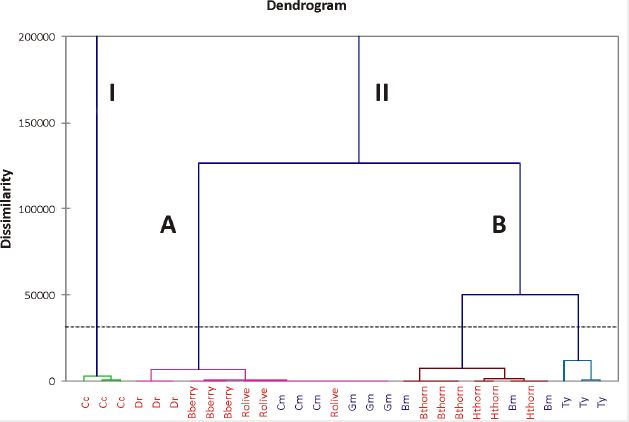
contents of OA and UA acids from HPLC profiles and the yield of extract. The results (Figure. 3) showed
that the investigated natural sources were divided into two main clusters (I and II) according to their
contents. Cornelian cherry samples were in cluster I and the other samples were in cluster II, which was
divided into two subgroups again (A and B). The results indicated that the samples with lower OA and UA
content, such as Dog Rose, Blackberry, Common Marigold, Ginger mint and Russian olive fruits, were
mostly classified in cluster A, while Blackthorn, Hawthorn, Bergamot mint and Thyme were grouped in
cluster B which is divided in two sub-clusters, corresponding to the different triterpenic bioactive content.
The determination results of 30 samples were further analyzed and classified by principal components analysis
(PCA). The first two principal components (PC 1 and PC 2) with 96% of the whole variances were
extracted for analysis. PC 1 accounted for 66.17% variances and PC 2 accounted for 29.83%. The other
principal components which had a minor effect on the model were discarded.
The scatter plot was shown in Figure 4, where each sample was represented as a marker (medicinal plants - blue and wild fruits - red). Generally an overlap was obtained for the majority of the investigated natural sources, except for Cornelian cherry, Thyme, Blackberry and Dog rose, with distinctive triterpenic bioactive content.
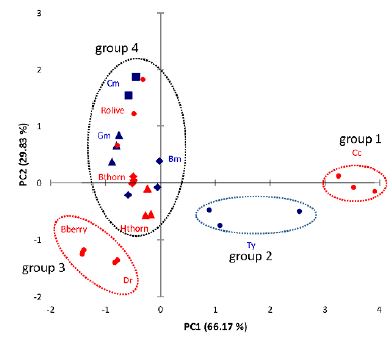
It was noticeable that the samples were clearly clustered into four groups: group 1 - Cornelian cherry, group 2 - Thyme, group 3 - Blackberry and Dog rose, representing the natural sources with high triterpenic content and group 4 - the unseparated natural sources which have a low triterpenic content.
Discussions
The two triterpenes OA and UA acids were present in all of the investigated natural sources. However, there
are significant quantitative differences between the different medicinal plants and wild fruits.
The OA contents in the obtained extracts varied from traces (Common Marigold (Calendula officinalis L.), Ginger mint (Mentha x gracilis ‘Variegata’) and Blackberry (Rubus fruticosus L.)) to 135.008mg OA/g of Romanian Tufted Thyme (Thymus comosus Heuff. ex Griseb.) extract and 165.857mg OA/g of Cornelian cherry (Cornus mas L.) extract. Other species with moderate OA levels were Dog rose (Rosa canina L.) (56.745mg OA/g extract) and Bergamot mint (Mentha piperita var. citrata (Ehrh.) Briq.) (28.259mg OA/g extract).
With the exceptions of two natural sources, Dog rose (Rosa canina L.) and Russian olive (Elaeagnus angustifolia L.), the levels of UA were always higher than those of OA in the tested samples. The UA contents ranged between traces (Common Marigold (Calendula officinalis L.), Ginger mint (Mentha x gracilis ‘Variegata’), Dog rose (Rosa canina L.) and Russian olive (Elaeagnus angustifolia L.) to moderate content for Bergamot mint (Mentha piperita var. citrata (Ehrh.) Briq.) (111.381mg UA/g extract), (152.786mg UA/g extract) and Romanian Tufted Thyme (Thymus comosus Heuff. ex Griseb.) (215.994mg UA/g extract) and high content for Cornelian cherry (Cornus mas L.) (522.797mg UA/g extract).
Our results showed that Cornelian cherry (Cornus mas L.), followed by Romanian Tufted Thyme (Thymus comosus Heuff. ex Griseb.), Hawthorn (Grataegus monogyna Jack.) and Bergamot mint (Mentha piperita var. citrata (Ehrh.) Briq.) represent valuable natural sources of triterpenic OA and UA acids. It can provide an ample opportunity to take these plants for mass cultivation in order to provide natural bioactive compounds that can be used as starting material for pharmaceutical development.
Further research studies with regard to more complex characterization of natural sources in terms of other triterpene acids (such as betulinic and maslinic acids) or phenolic and volatile compounds which contribute also to the overall therapeutic potential are highly recommended.
Conclusions
In the present study, the OA and UA triterpenic acids were simultaneously determined in different medicinal
plants and wild fruits extracts using a RP-HPLC–PDA method. The triterpene acids composition of the
obtained extracts depends on the plant material, Cornelian cherry (Cornus mas L.), Romanian Tufted Thyme
(Thymus comosus Heuff. ex Griseb.), Hawthorn (Grataegus monogyna Jack.) and Bergamot mint (Mentha
piperita var. citrata (Ehrh.) Briq.) extracts contain important amounts of OA and UA triterpenic acids.
HCA and PCA approaches applied on chromatographic data combined with the extract yield from the plant material allow to cluster the investigated natural sources and distinguish between them. The presented RP-HPLC–PDA method conjugated with HCA and PCA were proved to be very helpful methodologies for the classification of different medicinal plants and wild fruits.
Conflicts of Interests
The authors declare no conflict of interest.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!