Biography
Interests
Kulvinder Kochar Kaur1*, Gautam Allahbadia2 & Mandeep Singh3
1Scientific Director, Dr Kulvinder Kaur Centre for Human Reproduction, G.T.B. Nagar, Jalandhar, Punjab, India
2Scientific Director, Ex-Rotunda-A Centre for Human reproduction, Kalpak Garden,Perry Cross Road, Mumbai,
India
3Consultant Neurologist, Swami Satyanand Hospital, Ladowali Road, Jalandhar, Punjab, India
*Correspondence to: Dr. Kulvinder Kochar Kaur, Scientific Director, Dr Kulvinder Kaur Centre for Human Reproduction, G.T.B. Nagar, Jalandhar, Punjab, India.
Copyright © 2019 Dr. Kulvinder Kochar Kaur, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Monoterpenes are natural products from the terpenoid class and get synthesized with the use of mevalonic acid. They have small molecular weight and are nonpolar in nature, which, makes them the most large components of essential oils that are mostly considered to have some antioxidant and antimicrobial effects at quiet a large concentration. Recently they have been reported to have antidiabetic effects. In view of the indigineous biosynthesis that occurs in nature, they show lot of structural complexity and diversity that needs future structure activity analysis. In this review how monoterpenes may use different mechanisms for antidiabetic activity both by in vitro and in vivo animal studies is discussed. Both the aglycones and glycosides of these very small compounds show antidiabetic activity besides antiobesity and lipid lowering properties. Some of the important pharmacological targets include the insulin signaling pathway, and /or the associated PI3K/AKT, peroxisome prolifarator activated receptor gamma (PPARγ), glucose transporter 4 (GLUT-4) and adenosine monophosphate activated protein kinase (AMPK) pathway, proinflammato ry cytokines and the NFκB pathway besides myogenesis enhancement using the myogenic differentiation (Myo D) and myogenin (MyoG), and some like camphor and cystine based cyanopyrrolidines act as DPP-IV inhibitors like to control Type2 diabetes mellitus (T2DM).With a lot of natural products known to have anti insulin resistant effects need is there to probe further the role of these compounds alone or in combination with polyphenols as natural anti DM effects in view of rising incidence of diabesity and cost effectiveness and adherence of most antidiabetic drugs doesn’t allow their prolonged use.
Introduction
In the recent years various estimates of the current level of diabetes mellitus (DM) with its projection in the
next few decades has been projected. As per World Health Organization (WHO), there were a total of 422
million cases of diabetes mellitus (DM) in 2014 giving a 8.5% prevalence that increased from 4.7% in 1980
[1]. The major risk factor of DM has been recognized as obesity, whose prevalence was 600 million in 2014.
The number of adults (18 years or more) documented to be overweight were > 1.9 billion [2]. So much so
that the correlation of DM and obesity got termed “Diabesity” implicating the need to treat the 2 together
[3]. Now the consequences of calories than malnutrition has become a greater threat than undernutrition.
Currently DM is a cause of death in millions of people annually, and its impact has even more severity
with its association with other diseases like cardiovascular diseases (CVD), organ failure, like kidney and
disabilities like blindness and limb amputations [1].
No definite drug cure for DM exists and the therapeutic approaches consist of managing the disease, mainly the glycaemic control. DM manifests itself, when persistent hyperglycemia appears in blood which is secondary to insufficient amount of insulin released from the pancreatic β-cells and /or resistance to insulin is developed by vital organs. Diabetes type1 remains a most uncomplicated (T1D), where pancreatic βcells get destroyed via autoimmune mediated reaction. While Type2 DM (T2DM) is characterized by insulin resistance (IR), where biggest risk factors are age and obesity. This disease remains complex and might involve impaired insulin secretion and β-cell death along with different metabolic dysregulations.
Insulin that got discovered in 1920’s has been the mainstay for T1D and in uncontrolled T2DM [4]. i) α-glucosidase inhibitors like acarbose, miglitol and voglibose, target carbohydrate digestion in the gut =>limitation of the availability of glucose taken up by the blood, other groups of antidiabetic drugs used orally are, ii) The biguanides like metformin which suppress glucose release /production by liver iii) The thiazolidenediones like glitazones that increase the sensitivity of insulin target organs iv) The insulin secretagogue sulphonylureas and the meglitinides iv) The DPP-1V inhibitors v) The SGLT2 inhibitors vi) and the injectable glucagon like 1 peptide(GLP-1) analogues like liraglutide, semiglutide, among others [5]. These drugs have various side effects that include gastrointestinal side effects along with efficacy getting lost following prolonged use [6]. Further one has to consider high cost along with problems of patient compliance in view of long period of therapy and repeated dosage. Further in view of concept of diabesity one needs to use weight neutral drugs [3]. Hence need of the hour is finding novel antidiabetic drugs that have safer,more efficacious profile, and are cheaper. Since DM is a complex disease one needs to look for compounds which are multifunctional and target multiple mechanisms in view of diffuse pathology [7]. Further the antidiabetic effects of many natural products like polyphenols which combine antioxidant, anti-inflammatory, enzyme inhibition and different other insulin signaling modulatory effects have been described [8,9].
Methods
Thus a pubmed search along with Web Science, EMBASE were searched usingthe MeSH terms like
monoterpenes, iridoids, diabetes mellitus, natural products, biochemistry of these compounds along with
their biovailability from 1970-2019.
Results
We found a total of 251 articles pertaining to the topic of which we selected 106 articles for this review. No
meta-analysis was done. Thus this review is just dedicated to the biological activities mainly characteristic
of monoterpenes.
Biochemistry
Just like all terpenoids, monoterpenes, are constructed from repeating units of basic 5 carbon building
blocks called isoprenes [10,11]. Biosynthesis starts from the simple primary metabolite acetyl CoA that
goes through a series of biosynthetic pathways that involve the mevalonic acid. Hence, terpenoids are often
referred to as products of mevalonic acid pathway or secondary metabolites [12-14]. The conversion of
the primary metabolite into the secondary terpenoids, natural products involve the synthesis of the key
5 carbon reactive intermediates, isopentenyldiphosphate (IPP) and dimethyallyl diphosphate(DMAPP).
Condensation of these 2 isoprenes =>formation of a 10 carbon skeleton geranyl diphosphate (GPP):
the immediate precursor of all, monoterpenes (fig 1). Further isoprene units sequentially added to GPP
=>sesqueterpines (15 carbon) and diterpenes (20 carbon) or their dimers, the triterpenes (30 carbon) and
tetra terpenes (40 carbon) respectively. IPP and DMAPP, the 2 5 carbon terpenoid skeletons may =>some
5 carbon derivatives that are present in alcohol, acid, or hydrocarbon forms but they need to be incorporated
into other secondary metabolites as ether or ester derivatives. Thus the smallest but most structurally diverse
group of terpenoids are represented by the monoterpenes.
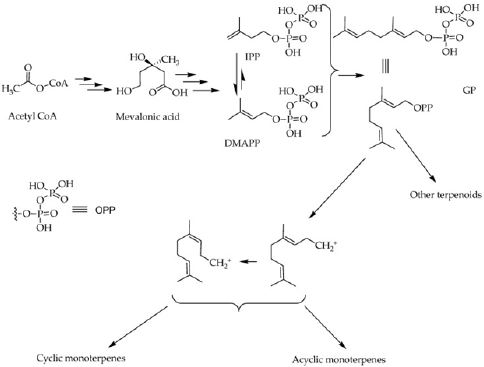
Inspite of their small atomic mass (7), the monoterpenes, have marked structural diversity in view of the diphosphate leaving group of the GPP.The cations which form as a result undergo a series of reactions that include that include addition reactions that typically =>incorporation of a hydroxyl group, or double bond rearrangements and unsaturation which all ultimately =>stable structures of the monoterpenes class (fig 2). The characteristic of monoterpenoids that arose from one precursor GPP (or its isomer neryl diphosphate) via a series of enzyme catalyzed reactions that include cyclization, hydroxylation, dehydrogenation, oxidation and/or reduction, isomerization, and conjugation have been the subject of over a century old research for the chemists doing natural research.
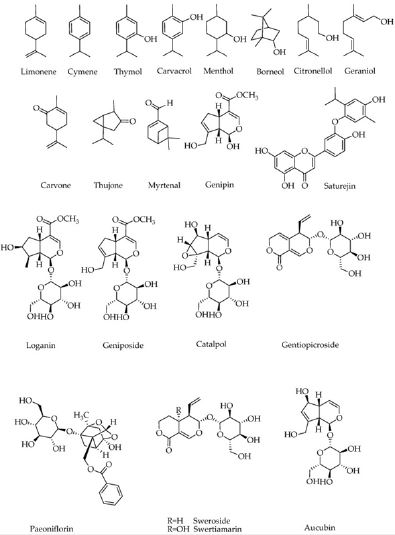
Most monoterpenoids, with their small molecular weight along with their nonpolar nature suggests that they het easily extracted by either non-polar solvents like hexane, supercritical CO2 or steam distillation. However some monoterpenes, exist in glycosylated form and hence remain very polar.
Regarding most monoterpenoids, the small molecular weight in addition to their nonpolar nature implies that they are easily extracted by either nonpolar solvents like hexane, supercritical CO, or steam distillation. However some monoterpenes exist in glycosylated form and hence are very polar. Of these the irinoids constitute such group of compounds having cis -fused cyclo-pentano pyran ring system in their structure (fig. 2) [14]. Further these monoterpenes might be incor porated into other structures like polyphenol (like phenolic acids and flavonoids).
Basic Function in Nature
Terpenoids get produced in various organisms including bacteria, fungi, plants and animals in whom they
play an important role. A big number of volatile compounds used by plants either to attract pollinator insects
or deter herbivores are the monoterpenes class of compounds. Mainly the good smell of herbs, spices, flowers
and fruits are given to essential oils that are mainly due to monoterpenes. Different roles of monoterpenes
are acting as signaling molecules in plant metabolism, plant to plant and plant to animal interactions have
been reviewed in detail [10,15-17]. Monoterpenes have been exploited for human use in perfume industry,
food industry, for flavoring and preservative potential of essential oils along with their antimicrobial and
antioxidant effects. Also their use as pharmaceutical agents [17-19] along within agrochemical industries
mainly as insecticides [20].
General mechanism of action as their use as antimicrobial and for cough therapy is mainly attributed to their volatile nature that allows them to move freely through space including biological membranes and interact with different biomolecules. Some of these actions have been known to be nonspecific, which lack drug like sensitivity to particular receptors. E.g. is the hydrophobicity of these compounds and essential oils in general have been shown to be responsible for their disruptive effect on bacterial cellular structures (e.g, cell membrane) and hence =>cell death [21]. Also non competitive and non specific mechanism of action have been described for muscle relaxant effect of monoterpenes. Like relaxant effect of carvacrol on tracheal smooth muscles of guinea pigs could not be accounted by effects via beta2 adrenergic stimulatory, histamine H1, and muscarinic blocking as shown by Boskabady and Jandaghi [22]. Conversely specific effects of monoterpenes on receptors, like the cooling effect of menthol via action on thermoreceptors is still subject of interest [23]. Also a noncompetitive manner of receptor inhibition by monoterpenes have also been reported like direct interaction of linalool with N-Methyl -D aspartate receptors [24] and nicotinic acetyl choline receptor inhibition by bormeoi [25].
Antidiabetic Effects of Monoterpenes
In vitro experiments on antidiabetic effects of monoterpenes are cell culture studies using pancreatic β-cells,
muscles, adipocytes and liver cells among others along with enzyme/protein based assays [26-39]. The study
by Tan et al [36] compared directly the number of commercially available monoterpenes, geraniol, nerol,
citral, (R)-(-) Linaool, (R)-(+)- Limonene ,S-(-) perrilyl alcohol, (R)-(+)-β-citronellol , S-(-)β citronellol,
α-terpineol, L-menthol, γ-terpinene and terpinplene. They used in vitro antioxidant, α-amylase and
α-glucosidase enzyme inhibition; glucose uptake and lipid metabolism in 3T3L-1 adipocyte. Though these
compounds have been shown to have some radical scavenging properties (DPPH and ABTS) radicals at
higher concentrations like 10 and 100mM, the observed action is not of therapeutic use. Considering their
structure (fig 1) that is lack of phenolic structural moiety (barring few exceptions), they are not expected
to display potential antioxidant activity and /or direct radical scavenging. Potent direct radical scavenging
effects of polyphenolic compounds especially those with catechol functional groups have been shown over
years and those having flavonoid skeleton [11,40-44]. Thus besides carvachol and thymol having phenolic
skeleton most monoterpenes don’t seem to have direct radical scavenging mechanisms which account for their antidiabetic effects. With regard to inhibition of crucial carbohydrate enzymes (α-amylase and
α-glucosidase) inhibition, these compounds were reported to act at 10mm [36] which is again not of
therapeutic use. In this connection many promising Natural compounds like flavonoids and polyphenols
act at micromolar ranges [9,41,43,44-47]. Most prevalent effects of these monoterpenoid compounds were
on glucose uptake and lipid metabolism in 3T3L-1 adipocyte cell cultures [36]. S-(-)β citronellol, (R)-
(-) Linaool and terpinolene did not effect the glucose uptake in 3T3L-1 adipcyte while geraniol, nrerol,
citral, (R)-(+)-Limonene, S-(-)perrilyl alcohol, (R)-(+)-β-citronellol , S-(-)β citronellol, α-terpineol, and
γ-terpinene showed some degree of activity (upto 21% inhibition)when tested at 1μM concentration.
Although this concentration is very small and promising, a dose dependent effect profile was not reported
for these compounds. Similarly the free glycerol released into the medium in cell culture was measured and
at 1μM concentrations, the effect of (R)-(+)-Limonene, S-(-) perrilyl alcohol, (R)-(+)-β-citronellol, and
geraniol were shown [36]. Again this is a screening result and doesn’t show the concentration range and
total profile of activity of these compounds. While (R)-(+)- Limonene did not affect the mRNA expression
of PPAR γ in 3T3L1 adipocytes, although it raised the mRNA expression of GLUT 1 by 12 fold while the
mRNA expression of GLUT 4 was unchanged. All of these were at fixed concentrations of one dose(1μM).
Even though essential oils are known for general antioxidant and enzyme inhibitory activities that include α-glucosidases, such effects may not be therapeutically relevant, in view of them occurring in high concentrations. Monoterpenes have a great diversity, although some like thymol are phenolic in nature while most are highly nonpolar compounds. Direct reactive oxygen species and radical scavenging (ROS), is mainly a function of polyphenolic compounds, that (with few exceptions like thymol and carvachol) are poorly represented in monoterpenes. Thus such biological effect is not discussed here as the major mechanism of these compounds for their antidiabetic effects. When monoterpenes get incorporated into other structural groups like flavonoids, as exemplified by saturejin, much better direct antioxidant and enzyme inhibitory effects were obtained [37]. To assess the effects of monoterpenes, number of other in vitro experiments were done on cultured pancreatic β cells. Remarkable results have been shown by these, since concentrations in micromolar range potentiated insulin secretion. Different experiments on hepatocytes, adipocytes and muscle cells also confirmed that these compounds can effectively increase glucose uptake via upregulation of the glucose transporter (GLUT4) translocation. Some critical pharmacological targets at molecular level that include the insulin signaling pathways have been involved.Genipin and geniposide along with iridoids and their glycosides also show potential antidiabetic effects in vitro. In the cultured muscle myotube cells, an increase in the phosphorylation of insulin receptor substrate -1(IRS1) and other signal transduction pathways =>to increased intracellular calcium concentrations have been documented [27]. The AMPK pathway has also been shown to be involved in the protection of pancreatic β cells by geniposide [28]. Further activation of glucagon like receptor-1 has been implicated in the insulin secretion promoted by geniposide [29]. Role of PPARγ and phosphatidyl-inositide-3 kinase(P13K) are also shown to be involved in the effects of these compounds in neuronal cells. PI3K involvement has been confirmed in the action of monoterpenes [31]. Thus at concentrations as low as 10μM, these compounds can protect pancreatic β cells and other cells like neurons; promoting insulin secretion and facilitate glucose uptake. Amelioration of proinflammatory cytokines like TNF in adipocytes and lipid accumulation in liver cells have been shown to be induced by geniposide. Involvement of GLP-1R signaling pathway in the antidiabetic effect of geniposide has also been confirmed by specific receptor antagonist extendin [32,33]. Production of TNF and free fatty acids(FFA’s) in adipocytes and macrophages in vitro could also be ameliorated by monoterpenes like paeoniflorin [10,11], swertiamarin [15] and thujone [16].
Inspite of their simple structure monoterpenes have shown promising antidiabetic effect in vivo [30,48-73].
List of monoterpenes having good effects on in vivo antidiabetic models including the streptozocin (STZ)-
induced diabetic, high fat diet (HFD)fed and spontaneously obese mouse models. Some of these compounds
are presented as simple hydrocarbons like limonene, cymene, ketone or hydroxy derivatives, aromatic or non
aromatic skeletons; or glycosides of iridoids (fig 2). Promising antidiabetic effect at doses as low as 5mg/kg
have been seen in all cases. Since variable dose regimens along with duration of study is there, it does not
allow direct comparison of potency between different structural groups of monoterpenes. Hyperglycemia was
the primary criteria that was assessed in all studies and the studied monoterpenes showed promising results
in all studied models. Besides lowering blood glucose levels, the common antidiabetic measurements involve
glycated hemoglobin measurements, primarily Hb A1c.In liver, the main antidiabetic drugs target,increasing
the level of glycogen levels was reported for borneil [49], citronellol [56] and myrtenal [65,66]. Modulation
of crucial enzymes of glucose metabolism like glucose-6-phosphatase and fructose-1,6, bisphosphatase
reduced glucokinase and glucose-6-phosphate dehyrogenase activities have been documented for cavacrol
[51], carvone [54], citronellol [56], geniposide [60], myrtenal [66] and paeoniflorin [53,69]. The common
markers of hepatic function aspartate aminotransferase (ASP), a lanine aminotransferase (ALP), alkaline
phosphatase(ALP), and gamma glutamyl transpeptidase have also been routinely measured in DM and their
increased status in DM gave been suppressed by carvachol [51] and carvone [54]. Other organ functions
primarily the kidney (cymene and logonin) and the brain (geniposide, thyroland D-limonene) have also
been documented.
The studied compounds on gross weight of animals specially when given to STZ induced DM is associated with wt loss. E.g .giving borneol [49], carvachol [50], and swertiamarin [70] have shown to reverse the body weight loss in DM. While, the body weight gain in HFD-obese model could get ameliorated by monoterpenes. Although catalpol had no effect at a dose of 100mg /kg though it improved fasting glucose and insulin levels [45], geniposide [59], paeoniflorin [58,69], and thymol were among the monoterpenes. which showed good potential antiobesity effect. Geniposide also showed antiobesity effects on spontaneously obese type 2 diabetic Tsumura Suzuki obese diabetic (TSCD)mice model [9].
Since STZ administration mainly targets pancreatic β cells =>insulin depletion and thus diabetic condition,insulin secretion is one of the main mechanisms of antidiabetic agents. Although carvacrol did not have significant effect on serum insulin level [53] carvacone [54], citronellol [56], geniposide [58], geranial [61], myrtenal [65] and swertiamarin [66], have been shown to increase insulin in the STZ induced DM. As shown for menthol [64] and myrtenal [66], improvement in the pancreatic and hepatic cellular and structures as evidenced from histological studies have been shown. On the other side, in the HFDobese model, hyperglycemia is associated with an increase in insulin level which appeared to be targeted / normalized by monoterpenes. Some good examples for these are geniposide [60] and thymol [73].
Antidiabetisc effects are measured usually by assessing the level of glycation of proteins which include hemoglobin that has been shown to be suppressed by monoterpenes. Seeing the link between glycation and oxidative stress, one should look at the antioxidant effects of these compounds in vivo. Although monoterpenes are not expected to have directly ROS scavenging effects in vitro, their in vivo antidiabetic effect is associated with an improved antioxidant status. Like borneol [49] has been demonstrated to increase antioxidant status in diabetic animals that include increased levels/activity of super oxide dismutase(SOD), catalase, reduced glutathione(GSH)in the liver and kidney, while concomitantly decreasing the level of oxidation marler, malondehyde (MDA). Similar results were obtained for aucubin [48], carvacrol [52,81], genipin [57], D-limonene [63], logonin [64], thymol [72].
In vivo the antihyperlipidemic and antidiabesity effects of monoterpenes is well demonstrated. The DM -induced increase in the plasma or tissue levels of TC, TGs, LDL-C, VLDL-C, are usually measured for assessing the potential lipid lowering effect of drugs, while increasing the levels of HDL is considered a valuable parameter of lipid modulation. Borneol [49], Carvacrol [53], genipin [57], geniposide [37], DLimonene [63], swetiamarin [74] and thymol [71,72] are classical examples of monoterpenes which show these effects. The accumulation of fat in tissues as seen with geniposide and paeoniflorin gave also been shown, besides the different effects in the HFD induced DM. Basically all these effects amrliorate DM inflammation caused by different monoterpenes, that include effects on proinflammatory cytokines like TNF along with the NFκB pathway.
Bioavailability
Liu et al studied the oral bioavailability of paeoniflorin in a single pass “four site” rat intestinal perfusion
model and cultured Caco-2 cells. Absorption of this compound in cultured cells was slower than in aglycone
(paeoniflorigenin). They found that poor permeation, P-gp mediated efflux, and hydrolysis via glucosidase
contributed to the poor boavailability of paeoniflorin. When the crude plant extract preparation pf Paonia
lactiflora roots (Chishao) which contain paeoniflorin as a major component (85.5%) was given in human
subjects by intravenous and multiple infusions, an elimination ½ lives of 1.2-1.3h was observed for paeoniflorin.
After it had been exposed to major organs, glomerular filtration based renal excretion had been seen to
be the main excretion pathway for this compound [75]. Bioavailability of paeoniflorin has been considered
to be low in rabbits (7.24%) and rats (3.24%) after oraladministration [76].
Oral administration of monoterpene aglycones like thymol and carvacrol has also been shown to have slow absorption in the blood stream [77]. Both unchanged and their glucuronide and sulfate conjugates have also been found. Like Dong et al [78] showed that cavacrol as a substrate to uridine -diphosphate glucuronyl transferase, can inhibit the enzyme. Another study that involved clinical trial involving 12 healthy volunteers, no thymol could be found in plasma or urine [79]. But its metabolites thymol sulfate and thymol glucuronide were found in the urine and the reported and the demonstrated mean terminal elimination ½ live was 10.2h. Further thymol sulfate was detected upto 41h after administration and urinary excretion normally followed over 24h [80].
In a study where potential anticancer therapy study, administration of limonene in women with newly diagnosed operable breast cancer showed preferential concentration of the compound in the breast tissue, reaching a high tissue concentration (mean of 41.3μg/g tissue) while its major active circulating metabolite was reported to be perillic acid [81]. The accumulation of limonene on adipose tissue(AT) has been reported in other studies [82]. Oral administration of swertiamarin in rats has been shown to be associated with rapid and wide distribution in tissues with the maximum amount obtained in liver and kidney, indicating, the perhaps the possible metabolism and /or elimination sites [83]. As shown for genopiside, administration of these compounds on the form of crude plant extract may be better for the bioavailability than the purified compounds [84].
More data is needed to establish a clear bioavailability and/or pharmacokinetic profile of monoterpene. That these compounds show antidiabetic effects when given orally and even at modest doses even at modest doses suggested that they can be absorbed/made bioavailable to exert their bio action to various organ systems. Although promising, further studies on optimization and formulation studies are very important. Since thay are extremely nonpolar, that implies that they may easily travel across cell membranes, but their poor water solubility remains a challenge, and considering this, getting an antidiabetic effect can be considered remarkable.Besides that glycosides of these compounds also show antidiabetic effects and might constitute another route of optimizing their antidiabetic effects.
Discussion and Conclusions
Inspite of structural simplicity of monoterpenes, in vitro and in vivo studies, show that they promise to
be considered as antidiabetic compounds either by their own or as part of structural moieties in complex
structures. Therefore different mechanisms of action given in these studies need to be probed further. There
is growing work showing PPAR γ plays a vital role in the regulation if carbohydrate, lipid and protein
metabolism both in healthy and disease states like DM, obesity and metabolic syndrome (MS), CVD,
etc. In case of DM the expression of PPAR γ with its predominant expression in adipose tissues, along
with macrophages and intestine has marked effects in the regulation of adipogenesis, insulin sensitivity
and inflammation [85,86]. Role of PPAR γ in crucial other insulin target organs /tissues like liver and
muscles is also well understood. Despite thiazolidenediones (TZD’S) group of antidiabetic drugs have been
reported to have numerous side effects [85,86], targeting these PPAR γ by receptor agonists is an attractive
pharmacological target both for prevention and treatment of metabolic disorders that includes DM. Thus
role of natural products as a source of potential drugs which don’t gave these side effects of TZD’s has been
recommended [87,88]. Thus the modulatory effect of monoterpenes on PPAR γ appear to be a well defined
mechanism of antidiabetic action. Moreover, carvachol, thymol and other monoterpenes, which showed
antidiabetic effects are commonly found in foods and flavors and thus not expected to show side effects of
TZD’s.
Role of AMPK in controlling glucose metabolism as a target for DM has received a lot of attention in these past years. Drugs activating AMPK, enhance the phosphorylation of insulin receptor substrate 1(IRS1) Ser 789 phosphorylation =>the cascade of activation of phosphoinositide 3kinase /protein kinase B (PI3K/ PKB) signaling [89]. The classical antidiabetic drug metformin, also appears to act by activation of the AMPK pathway in different target organs [90]. Activation of AMPK in adipocytes suppresses lipogenesis while promoting energy loss which suggests potential antiobesity effect [91]. Though some studies demonstrate paradoxical nature of AMPK activation of glucose and lipid metabolism mostly related to the level of stimulation (chronic vs acute [92,93]. Role of AMPK activators in different disease conditions is well known [94] Antidiabetic potential of natural products like anthocyanins through effects on the AMPK and insulin signaling pathways were highlighted [94]. Monoterpenes share this key mechanism of antidiabetic effect.
GLUT 4 represents an ATP-independent glucose transport system across all cell membranes and is mainly expressed in AT’s and skeletal muscles. Under expression of GLUT 4 can predispose animals to DM and IR, its overexpression overcomes this physiological/pathological disorder [95]. Decreased level of GLUT4 has also been seen in muscles of T2DM patients. The coupling between insulin receptor activation in target organs with the known action of insulin in GLUT4 -mediated glucose uptake has been the subject of lot of research recently. The insulin receptor substrate proteins phosphorylation =>activation of PI3K which in turn =>generation of phosphatidoyl inositol 3,4,5 -trophosphate(PIP3).PIP3 in turn is linked to recruit protein Kinase B(PKB), also called Akt, which plays a crucial role in the insulin dependent GLUT4 mobilization [96]. Metformin, which modulates the AMPK pathway affects the translocation of GLUT4 in insulin target cells [97]. Thus agents that facilitate the expression/mobilze GLUT4 could have antidiabetic effects via upregulation of GLUT4 expression and or/translocation. The modulation of the AMPK pathway by natural products which enhance glucose uptake that concomitantly inhibit neogluconeogenesis and stimulates glycogenesis have been documented [98-100].Thus multiple mechanisms involving insulin signaling and other pathways involving AMPK, GLUT4 and PPAR γ play role in the antidiabetic effects of monoterpenes.
Liver has a central role in glucose homoestasis by regulating glucose production and storage in the formation of glycogen. For drug therapy, agents which suppress the glycolysis pathway (glycogenolysis inhibitors) or hepatic glucose production(gluconeogenesis) have antidiabetic properties [101]. Classical examples of antiDM drugs therapy target key enzymes involved in the cascades of reactions in hepatic cells. Most important target is the 1st step of glycogenolysis, which requires the action of enzyme glycogen phosphorylase (GP) to convert glycogen to glucose-1-phosphate. Reversely glucose-6 phosphatase (G-6 Pase) at the final step of gluconeogenesis acts as an important target. Effect of monoterpenes as modulators of these pathways have been reported. Thus antidiabetic effects of monoterpenes include both gluconeogenesis and glycolysis as potential targets.
Importance of inflammation as a link between T2DM and obesity has been well documented [102,103]. This low grade persistent/chronic state of inflammation in obesity is believed to increase the IR in target organs [104]. This close link between inflammation under obese condition and IR of T2DM has become closer with the identification of proinflammatory cytokines like TNF-α and interleukin -6 (IL-6) as the major perpetrators [105]. E.g. PI3K-AKT (or PKB) pathway of insulin signal ing pathways can be inhibited by these cytokines via NFκB. This crosstalk between inflammation pathway and obesity/T2DM as mechanism of actions for natural products like anthocyanins has also been documentesd [94]. Since different monoterpenes suppress inflammation associated with T2DM, partly their therapeutic activity can be related to this pharmacological effect. Along with these multiple mechanisms, the lipid reducing and / or antiobesity effect have been shown for monoterpenes. The direct effect of monoterpenes on lipogenic expression by targeting crucial enzymes required for fatty acid synthesis (acetyl Co A carboxylase and fatty acid synthase) have been shown in vitro [10].
Along with these multiple mechanisms of action monoterpenes also have specific action by modulation of the GLP-1 receptors. The best studied compound remains the iridoid glycoside genoposide [38]. This mechanism has been confirmed for genoposide by using the receptor antagonist extend in [32,33].
Further Xu et al gave a new hypoglycaemic method of catalpol by enhancing myogenesis, as shown by increased myogenic differentiation (MyoD), Myogenin (MyoG) and myosin heavy chain(MHC). Although both metformin and catalpol increased glucose uptake by activation of PI3K/AKT pathway, unlike metformin, PI3K/AKT Pathway activation was dependent on increased MyoD/ MyoG mediated myogenesis [106].
Kuranov et al evaluated camphor and cystine based -cyanopyrrolodines as DPP-IV inhibitors for the treatment of T2DM.They found bornyl based cyanopyrrolodines had moderate inhibitory activity with regard to DPP-IV (1.27-15.78 μM. The in vivo hypoglycaemic activities were tested using oral -glucose tolerance test (GTT). bornyl based cyanopyrrolodines had good hypoglycaemic activity [107].
Thus concluding that both in vivo and in vitro studies regarding antidiabetic effects of monoterpenes have shown promise both for antidiabetic effects along with their possible antiobesity and lipid lowering agents. The problem is still clinical human work has not been done. These compounds being structurally simple, they appear to have a good set of actions with more opportunity to optimize their activity using structure activities. This approach is still not done as they are newer agents in the field of antidiabetic agents. On incorporation of these compounds with other structural groups like flavonoids [37] remains an interesting development. Flavonoids possess potent antioxidant effect which is of importance for amelioration of both diabetes induced glycation and oxidative injuries. Flavonoids along with other phenolic compounds have α-glucosidase inhibition along with other antidiabetic mechanisms. Formation of monoterpenes is another way by which they can exert antidiabetic actions. Though their marked nonpolar nature usually gets compensated by glycosylation, further studies are needed to modify their bioavailability and general pharmacokinetic profiles. Regarding this potential in silico studies for prediction and optimization needs to be used. Example of index model on filtering and mapping discriminative physical properties for finding antidiabetic potential of natural products has also been documrented [108]. With these waiting developments, current evidence suggest that monoterpenes are promising antidiabetic lead compounds via multiple mechanisms as shown above. Further Abbasi et al tried to study the inhibiting mechanism of thymol on xanthine oxidase, which catalyzes xanthine into uric acid, using the electrochemical technique coupled with the four way parallel factor analysis (PARAFAC). To complement this inhibitory effect ultraviolet and visible (US-Vis spectroscopy and molecular docking studies revealed that thymol could enter the catalytic centres of XO. Further it inhibited XO activity by directly binding to flavin adenine dinucleotide(FAD) center. These dose dependent inhibition of XO with thymol was linked to its antioxidant properties to reduce the formation of free radicals and related diseases [109,110].
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!