Biography
Interests
Solís Pacheco, J. R., Pulido Mateos, E. C., Rodríguez Arreola, A., González Reynoso, O., Balcázar López, E., González Quezada, E., Córdova López, J. & Aguilar Uscanga, B. R.*
University Center of Exact Sciences and Engineering, University of Guadalajara, Department of Pharmacobiology, Industrial Microbiology Laboratory, México
*Correspondence to: Dr. Aguilar Uscanga, B. R., University Center of Exact Sciences and Engineering, University of Guadalajara, Department of Pharmacobiology, Industrial Microbiology Laboratory, México.
Copyright © 2018 Dr. Aguilar Uscanga, B. R., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Ammonia is a toxic metabolite capable of causing hepatic encephalopathy in people with liver problems. This is a syndrome characterized by a series of clinical manifestations derived from attention deficit as well as cognitive deterioration, joined with the risk of fulminant hepatitis or liver cirrhosis caused by a significant reduction of hepatic parenchyma. The applications of probiotics and prebiotic in functional food is increasingly important in the prevention of diseases as the hepatic encephalopathy. Nevertheless, there is a little or null evidence of study about ammonium consumption by probiotic bacteria, in a fermentative system. Therefore, the aim of this work is to analyze the capacity of Lactobacillus rhamnosus HN001 and Bifidobacterium lactis Bi-07 (commercial strains), for consuming ammonium in different culture media and at different concentrations of ammonium sulfate (7 and 20g/L), using two carbon sources (glucose and fructan) for producing biomass. The results show that the bacteria are able to grow in different conditions and culture media. However, not all of the ammonium sulfate was consumed by bacteria. This research allows verifying the null capacity of these probiotic bacteria for ammonium consumption, but not to fructan consumption as source of carbon, so we consider that this in vitro study is important, considering that, the hypotheses established by other authors show a beneficial effect to people with liver encephalopathy problems.
Introduction
The beneficial effects of probiotic bacteria have been well-established in the treatment of gastrointestinal
diseases and also have the potential to reduce the risk of developing inflammatory bowel diseases, they
activate of the immune system, prevention of cancer cell growth, maintenance of mucosal integrity and
promote of an antagonistic environment against pathogens [1-3]. The anticancer activity through induction
of apoptosis of cancer cells seems to be promising approach for use of some probiotic strains as a support
therapy or this disease prevention. Nevertheless, in vivo studies are necessary to ascertain if results obtained
experiments from in vitro can be translated to the clinical practice [4].
New evidence suggest that probiotics affect beneficially the intestinal microbiota influencing in gut permeability, systemic inflammation levels preventing the production and/or uptake of lipopolysaccharides in the gut [5], and therefore reducing levels of low-grade inflammation, and host metabolism, thereby contributing to obesity [6] and fatty liver disease [7-9]. The probiotics they act by modulating visceral and hepatic fatty deposition via the gut-liver axis. Consequently, they may be proposed as add-on non-alcoholic fatty liver disease (NAFLD), treatment complementary to standard dietary and behavior strategies [10,11]. Non-alcoholic fatty liver disease (NAFLD) is the hepatic manifestation of metabolic syndrome and the leading cause of chronic liver disease in pediatric and adult individuals living in industrialized countries [10,12,13].
Modulation of gut microbiota by probiotics is supported by a number of studies conducted with nonalcoholic fatty liver disease animal models and in several pilot pediatric and adult human studies. Globally, this approach appears to be a promising add-on therapeutic tool to be used in the context of a tailored multi-target therapy especially in cases where standard dietary and lifestyle changes have failed. The hepatic fat metabolism also seems to be influenced by the presence of commensal bacteria, and potentially by probiotics [11]. Although the mechanisms by which probiotic might act on the liver are still unclear. However, this could be of great importance in the future because the infiltration of liver fat and hepatitis may be more prevalent as a result of high fat consumption, and may cause liver failure complicating hepatic encephalopathy [7].
Xu et al (2012) [14] comparing two probiotics (Lactobacillus acidophilus and Bifidobacterium longum) in rats with HFD induced NAFLD, Bifidobacterium longum was superior in attenuating liver fat accumulation. The lack of changes in intestinal permeability in treated mice was attributed to the effect of peptidoglycanpolysaccharide polymers rather than to endotoxin-induced stimulation of TNF-α release. This concept is supported by a human study in which levels of antibodies to peptidoglycan polysaccharide polymers significantly decreased after administration of Lactobacillus GG in pediatric NAFLD [15]. In the other hand, a treatment with Bifidobacterium longum plus the prebiotic (FOS) induced a significant improvement in serum inflammatory, metabolic, and liver enzyme parameters. End-of-study repeat liver biopsies showed improved fibrosis scores in 70% of patients and a decrease in the NASH activity index [16].
Probiotics, like other bacteria, have a set of molecules known as molecular patterns that interact with specific recognition receptors, present on the surface or membrane of the organelle of epithelial and dendritic cells. This interaction is responsible not only for some of the beneficial effects attributed to probiotics, but also for the normal interaction of the gut microbiota with the human host [17]. To be considered a probiotic bacterium it must have a high capacity of adhesion to the intestinal epithelium, produce antimicrobial substances such as bacteriocins and short-chain fatty acids, inhibiting the growth of potential pathogens [16].
Hepatic encephalopathy (HE) is a common neuropsychiatric manifestation in liver diseases. A dysfunctional liver is unable to purge ammonia from the bloodstream, which builds up to toxic levels in the central nervous system [18]. Certain studies to show the effectiveness of consumption of probiotics in improving HE, have used probiotic bacteria such as Lactobacillus acidophilus, Enterococcus lactis, Lactobacillus paracasei subspecies paracasei, Lactobacillus plantarum, Lactobacillus rhamnosus GG and Bifidobacterium lactis Bi-07 [19-21]. However, knowledge about the regulation of nitrogen metabolism in lactic acid bacteria is limited. Bajaj et al., (2008) [21] analyzed twenty-five patients and 17 yogurt marks. The patients demonstrated a significant improvement in the number of connection test-A (NCT-A), block design test (BDT), and digit symbol test (DST) compared to baseline/no Rx group. No adverse effects or change in covariates was observed. This trial demonstrated a significant rate of HE reversal and excellent adherence in cirrhotic after probiotic yogurt supplementation with potential for long-term adherence.
Lactobacillus rhamnosus HN001 is a well-characterized probiotic strain, identified by Prasad et al. (1998) [22], in the New Zealand Dairy Research Institute, and was selected in studies in animals and humans for their ability to survive at low pH and relatively high concentrations of bile, actually is marketed by the company DANISCO [23]. In order to evaluate the safety of Lactobacillus rhamnosus HN001 several authors have worked supplying different doses of these bacteria to test mice. Shu et al. (1999) [24], provided Lactobacillus rhamnosus HN001 (5x107, 5x109 or 5x1010 CFU/mouse/day) to mice for 7 days. There were not abnormal clinical signs or differences in food intake, water or weight gain compared with a controlled group (no probiotic supplement). L. rhamnosus HN001 was not detected in the spleen of animals.
Good et al., (2014) [25], they showed that oral administration of live or UV-inactivated Lactobacillus rhamnosus HN001 attenuates Necrotizing enterocolitis (NEC) severity in newborn mice and premature piglets, as manifest by reduced histology score, attenuation of mucosal cytokine response, and improved gross morphology. Strikingly, DNA of Lactobacillus rhamnosus HN001 reduced the extent of proinflammatory signaling in cultured enterocytes and in samples of resected human ileum ex vivo, suggesting the therapeutic potential of this probiotic in clinical NEC. Taken together, these findings illustrate that Lactobacillus rhamnosus HN001 is an effective probiotic for NEC via activation of the innate immune receptor TLR9 and that Lactobacillus rhamnosus DNA is sufficient for its protective effects, potentially reducing concerns regarding the infectious risk of this novel therapeutic approach.
Loguercio et al. (1987) [19] conducted a pilot study in patients with hepatitis of different causes (HCV, alcoholism, and NASH), which received a probiotic mix (Lactobacillus acidophilus, L. bifidus, L. rhamnosus, L. plantarum, L. salivarius, L. bulgaricus, L. lactis, L. casei, L. breve, more fructooligosaccharides and vitamins). Interestingly, the authors reported that the strongest effect of probiotics was seen in patients with alcoholic liver cirrhosis, where all parameters of liver function improved, as happened with TNF-α and malondialdehyde. From these studies in humans, it appears that the microbiota is an important cofactor in the etiology of chronic liver diseases, and that probiotics might have a therapeutic role.
On the other hand, another group of probiotic bacteria are Bifidobacteria which are natural inhabitants of the human gastrointestinal tract and from different animals, which are present in varying amounts throughout life, appearing a few days after birth [26]. The beneficial intestinal flora, represented mainly by the genera Lactobacillus and Bifidobacterium, contributes significantly to the health status of the host, by its functions: (i) metabolic, intervening in the assimilation of nutrients from the diet; (ii) protective, contributing to the barrier effect and to the displacement of pathogenic microorganisms, and (iii) trophic, intervening in the modulation of the immune system and in cell development and proliferation [5,27].
Bifidobacterium lactis is of human origin, were discovered in the faeces of breast-fed infants, causing special interest to scientists as these bacteria are typically the most abundant species in the intestine of breastfed infants and regarded as a primary reason for the infants’ greater resistance to disease. These bacteria were originally described by Meile et al., in 1997 [28] and was later re-classified as B. animalis subsp. lactis [29]. Bifidobacterium lactis Bi-07 is marketed by the company DANISCO and was deposited at the “American Type Culture Collection” as SD5220. Bifidobacterium lactis Bi-07 has been properly classified as Bifidobacterium lactis, using modern genotypic methods, including the sequencing of the rRNA 16S gene [30]. Today bifidobacteria are broadly recognized for their key role in the human intestinal microbiota throughout life. A high proportion of bifidobacteria in the intestinal tract is considered beneficial to health.
Some species as Bifidobacterium longum, B. bifidum are capable to use as only nitrogen source, ammonium salts, and when grown in the absence of a source of organic nitrogen, secrete large amounts of amino acids such as threonine, alanine, valine and aspartic acid, resulting in inhibition of the synthesis of harmful products including ammonia [31].
Deguchi et al. (1993) [32] selected two B. bifidum strains that prefer to use ammonia than any other organic nitrogen compounds by measuring incorporation of the stable isotope ammonia by the cells. They estimated that fermentable carbohydrates might enlarge the caecum and enhance a blood flow in the aorta around the caecum, resulting in a large flux of urea to the caecum and higher absorption of ammonia into the portal vein. This increased level of ammonia was reduced by oral administration of B. bifidum YIT4069, suggesting that B. bifidum YIT4069 must assimilate ammonia in the caecum. The oligofructose has a stimulating effect on the growth of bifidobacteria, which are metabolized by bacteria as they are not degraded by human digestive enzymes [33].
Due to the ability of probiotics to consume ammonium, the effect of the concentration of carbon and nitrogen respect to survival of Lactobacillus rhamnosus HN001 and Bifidobacterium lactis BI07, was studied. These bacteria were grown in synthetic media (YPD and YPF), supplementing with ammonium sulfate, in order to observe the kinetic behavior and consumption of ammonium of these bacteria.
Materials and Methods
Lactobacillus rhamnosus HN001 and Bifidobacterium lactis Bi-07 were isolated from DuPont™ Danisco®
commercial probiotic cultures. For long-term maintenance these bacterial strains were stored lyophilized in
a -80°C freezer.
The MRS broth (BD Difco, USA) was rehydrated according instructions of manufacturer. YPD broth media
contained 2g of bovine casein peptone (BD Bioxon, USA), 5g of yeast extract (BD Bioxon, USA), 1g of
dipotassium phosphate (JT Baker, USA), 20g of dextrose (BD Difco, USA) and 7 or 20g of ammonium
sulfate (Baker Analyzed, USA) per liter of culture media. YPF broth media contained 2g of bovine casein
peptone (BD Bioxon, USA), 5g yeast extract (BD Bioxon, USA), 1g of dipotassium phosphate (JT Baker,
USA), 20g of fructans from Agave tequilana Weber (Fructoreal, MEXICO) and 7 or 20g of ammonium
sulfate (Baker Analyzed, USA). YPD and YPF culture media were prepared by suspending all the ingredients
in 1L of distilled water and boiling until the ingredients were completely dissolved. The pH of the mixture
was adjusted with NaOH 1N and HCl 1N to 7 ± 0.1. The mixtures were autoclaved at 121°C for 15 min
and cooled to 37°C prior to use.
To carry out the fermentation kinetics, the lyophilized strains were reactivated in 50mL of sterile MRS
broth. The flasks were incubated at 37°C, without agitation for 32h. Subsequently the fermented media
were transferred to sterile 50mL Falcon tubes and centrifuged at 3500rpm for 5min. The supernatants were
discarded and the recovered biomass was added to an Erlenmeyer flask containing 200mL of each tested
medium. Thereafter, to obtain exponential-phase cells, the flasks were incubated at 37°C, without agitation
for 15h.
Fermentation kinetics of L. rhamnosus HN001 and B. lactis Bi-07 in MRS broth were performed in duplicate
in Erlenmeyer flasks containing 500mL of culture medium. Flasks were inoculated with the recovered
biomass from the inoculum, adjusting to an optical density (OD) of approximately 0.3. Fermentations were
performed at initial pH of 7 at 37°C and without agitation. During exponential growth phase, a sample of
10mL was taken out every two hours to measure cell growth (biomass), substrate utilization (dextrose, agave
fructans, ammonium and protein) and metabolites production (organic acids).
Fermentation kinetics of L. rhamnosus HN001 and B. lactis Bi-07 were performed in duplicate in Erlenmeyer
flasks containing 500mL of YPD broth (2g/L of bovine casein peptone, 5g/L yeast extract, 1g/L of dipotassium phosphate and 20g/L of dextrose) and for YPF medium (2g/L of bovine casein peptone, 5g/L
yeast extract, 1g/L of dipotassium phosphate and 20g/L of fructans from Agave tequilana Weber) additional
7 or 20g/L of ammonium sulfate respectively. Flasks were inoculated with the recovered biomass from inoculum,
adjusting to OD approximately 0.3. Fermentations were performed at initial pH of 7 at 37°C without
agitation. During exponential phase, a sample of 10mL was taken out every two hours to measure cell
growth (biomass), substrate utilization (dextrose, total protein and ammonium) and metabolites production
(organic acids).
Biomass was determined by a calibration curve relating optical density to cell density (evaluated as dry
weight) for each bacteria and each tested medium. Optical density was measured at 600nm using a Jenway
6305 spectrophotometer. 1ml culture sample was taken out from the exponential phase and then diluted to
OD lower than first one. Dry weight of bacterial cells was obtained by centrifuging at 3500rpm for 5min,
1mL culture sample placed in Eppendorf tubes previously dried to a constant weight. After removing the
supernatant, the tubes were located in a hermetically sealed oven at 70°C for 24h and subsequently put in a
desiccator containing dried silica gel for 15min. The tubes were weighed in analytical balance (AE ADAM
NBL 84e), the dry weight was recorded and the dry biomass concentration was calculated using the following
formula:
Where:
PTB is the tube weight with dry biomass
PTV is the weight of the empty tube
A Varian ProStar HPLC with a Refractive Index Detector (VARIAN model 356-LC) was used for measuring
substrate consumption and metabolite production, including glucose, fructose, ammonium sulfate, butyric
acid, propionic acid, lactic acid, acetic acid and ethanol. A Varian MetaCarb H Plus column of 300mm ×
7.8mm was used for analytical separation. The adopted chromatographic conditions were: mobile phase
of 0.01N sulfuric acid solution (prepared with HPLC grade water), flow rate of 0.6mL/min, temperature
of 54°C and an injection volume of 20μL. A calibration curve was performed using a standard solution
containing 2g/L glucose (Difco, USA), 2g/L of fructose (Sigma, USA), 2g/L of ammonium sulfate (Baker
Analyzed, USA), 8g/L of lactic acid (Sigma, USA), 1g/L of acetic acid (Reasol, USA), 1g/L of propionic
acid (Sigma, USA), 1g/L of butyric acid (Aldrich, USA) and 1g/L of ethanol. The following concentrations
of sulfate, glucose and fructose were prepared and used for the calibration curve: 4, 8, 10, 12, 16 and 20g/L.
For lactic acid, the concentrations were the following: 0.2, 0.4, 0.5, 0.6, 0.8, 1g/L. Finally, for acetic acid,
propionic acid, butyric acid and ethanol, the concentrations were the following: 0.2, 0.4, 0.5, 0.6, 0.8 and
1g/L.
The ammonium concentration was determined according to the method described by Weatherburn (1967)
[34]. For the analysis, two reagents were prepared; reagent “A” was prepared dissolving 5g of phenol and
25mg of sodium nitroprusside (SIGMA, USA) in distilled water at 500mL volume was reached. Reagent
“B” was prepared dissolving 4.2mL of sodium hypochlorite (Golden Bell, USA) and 2.5g of sodium hydroxide
(JT BAKER, USA) in distilled water at 500mL. After preparing the reagents, each 20μL sample was
treated with 5mL of reagent “A” and 5mL of reagent “B” and subsequently incubated for 30 min at 37°C. The
optical density was measured at 625nm using a Jenway 6305 spectrophotometer. A calibration curve was
performed for each assay using a standard solution (1.8g/L) of ammonium sulfate (Baker Analyzed, USA)
for preparing the following concentrations: 0.1, 0.3, 0.5, 0.7, 1.2 and 1.8g/L.
The consumption of protein was determined according to the Lowry et al. method (1951) [35]. For the analysis,
each 100μL sample was treated with 1mL of Lowry reagent and subsequently incubated for 30minutes at
room temperature. After incubation, 100μL of Folin reagent (diluted 1:1 with distilled water) was added to
the tubes containing the samples which were vortexed and incubated for 30minutes at room temperature.
The optical density was measured at 595nm using a Jenway 6305 spectrophotometer. A calibration curve
was performed for each assay using a standard solution (1g/L) of bovine serum albumin (Equitech-Bio-Inc,
USA) for preparing the following concentrations: 0.1, 0.2, 0.3, 0.5, 0.75 and 1g/L.
A 22 full factorial experimental design with two replications was used to study the influence of Agave
Tequilana fructans and ammonium concentration (in YPF and YPD culture media) on kinetic parameters
of two probiotic bacteria. The response variables were the following: produced biomass, maximum growth
rate, substrate consumption rate and yield. The controlled factors were the following: type of carbon source
(glucose or agave fructans) and ammonium sulfate concentration (7 and 20g/L). Statistical analysis of data
was performed through analysis of variance (ANOVA) using Statgraphics Centurion XV Software. Results
are expressed as means ± S.D. (standard deviation), the calculations were performed using Excel 2007
(Microsoft, Redmond, WA, USA) program.
Results and Discussion
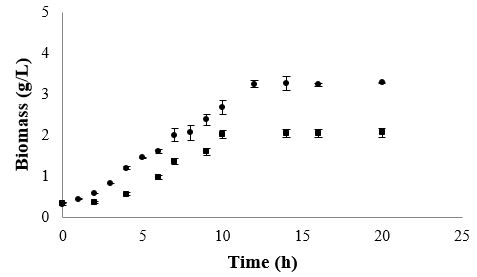
Fermentations in MRS broth were carried out in order to meet the growth kinetic parameters of the studied
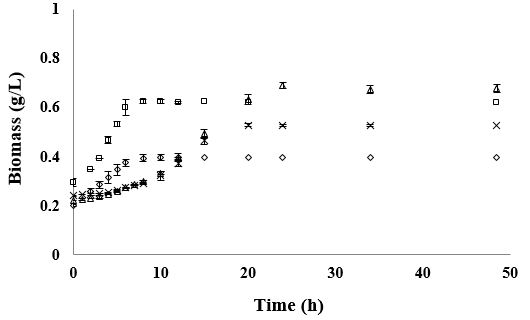
bacteria under no nutritional stress conditions. In this medium L. rhamnosus and B. lactis grew exponentially
during 10 to 11h (Figure 1). However, L. rhamnosus showed a maximum growth rate (μmax) of 0.32h-1 and a
generation time of 2.2h, achieving at the end of its exponential growth phase a maximum biomass content of 2.97g/L.
According results, the amount of ammonium sulfate is capable of reducing the growth rate of L. rhamnosus HN001, whereas the fructan, as carbon source, is able to promote it. Regarding the increase in the growth rate due to the presence of fructan, it is noteworthy that these results are similar to those obtained by Salminen et al. (2006) [33] which reported that some heterofermentative lactobacilli exhibit a faster growth rate with fructose than with glucose. It is important to mention that in this work did not use fructose as carbon source, but a polysaccharide consisting mostly of fructose with glucose in a ratio of 8:1.
Lactobacillus then had to break fructan molecule through an enzyme β-1,2-β-fructofuranidase for getting the monosaccharides contained in the molecule. Fructose is capable to play a role both as a substrate for growth as the electron acceptor; and it is fermented by 6-PG/PK, but part of the carbohydrate are reduced to mannitol by a NAD+ dependent mannitol dehydrogenase enzyme [36]. This allows to cells to use ATP via acetate kinase reaction. This way is less efficient that fermentation of glucose in terms of ATP formed per mole of consumed carbohydrate. However, L. rhamnosus, being heterofermentative lactobacillus, could have used glucose as an energy source and fructose as an electron acceptor, thus obtaining a higher growth rate, similar to reported by Gobbetti et al. (1995) [37].
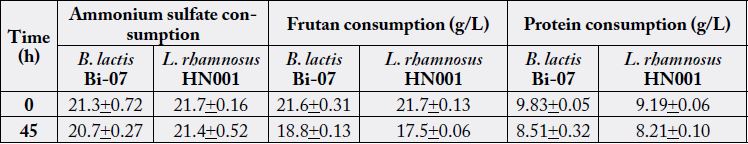
Whereas in the same culture conditions, B. lactis was able to grow at a μmax of 0.26h-1, showing a generation time (GT) of 2.6h and achieving at the end of its exponential growth phase a maximum biomass content of 2.14g/L. The growth of both bacteria was directly correlated to their substrate utilization, obtaining a substrate consumption rate (qs) of 2.7g/g*h-1 by L. rhamnosus and 2.6g/g*h-1 by B. lactis; indicating that these bacteria consumed approximately 3g of glucose per h to support their rapid growth. The yield of conversion of glucose into biomass was 0.12g/g-1 by L. rhamnosus and 0.10g/g-1 by B. lactis. Moreover, glucose was almost completely exhausted after both microorganisms reached their stationary phase. On the other hand, proteins were not completely utilized during 24h of fermentations (Table 1), observing 66% of its consumption by B. lactis and 41% by L. rhamnosus.
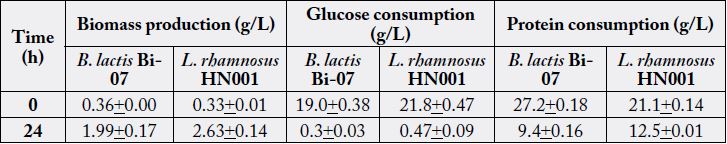
* The values in the table represent the mean and standard deviation +/- of three assays (n=3).
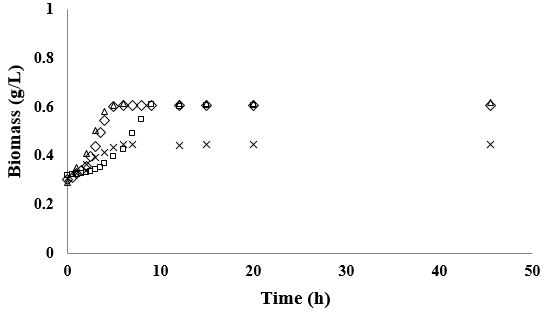
The results of the fermentation kinetics of L. rhamnosus in YPF and YPD media supplemented with 7 and
20g/L of AS are shown in Figure 2. In these media, L. rhamnosus tended to grow exponentially for about 5
to 6.5h. Furthermore, its μmax was greatly influenced by the AS concentration (P>0.0000), observing faster
growth when it was cultured in the media with the lowest AS content (Table 1). The type of carbon source
also influenced the rate in which this microorganism grew (P>0.0000), noting in the 45hour fermentation
that AF had a positive effect on it (Table 2 and 3). Additionally, a statistical interaction was found between
both factors (P=0.0445), where L. rhamnosus had greater growth rates when it had in its media 7g/L of
AS and AF as carbon source (μmax 0.21h-1). The GT was also affected by these factors, observing lower GTs
(3.23 and 3.98 versus 6.22 and 8.96h) in the fermentations performed with the lowest AS supplementation
(7g/L).
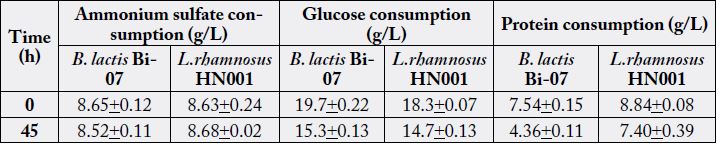
* The values in the table represent the mean and standard deviation +/- of three assays (n=3).
* The values in the table represent the mean and standard deviation +/- of three assays (n=3).
In these fermentations carbon sources were not completely utilized, observing a residual glucose content approximately 79% in the fermentations performed in YPD medium, and approximately 82% of residual fructan content in the ones performed in YPF (Table 3). The AS content significantly affected the rate in which L. rhamnosus consumed its carbon source, observing higher qs (2.29 to 2.83 versus 1.52 to 1.93g/g*h-1) with the lowest AS supplementation (7g/L) (P=0.0019). The yield of conversion of glucose into biomass by L. rhamnosus in these media ranged from 0.04 to 0.08g/g–1, showing relatively low yield compared to results obtained with MRS medium. Interestingly, in these media L. rhamnosus showed a preference for utilizing protein over ammonium as nitrogen source, observing 9 to 15% of protein consumption, and nearly 0% of ammonium utilization.
It is also important to mention that in the kinetics of L. rhamnosus HN001 on YPF medium with 7 and 20g/L of ammonium sulfate, a similar amount of biomass was obtained. However, in the case of the kinetic with 20g/L of ammonium sulfate, an increased generation time was observed because the biomass production increased at lower speed. In MRS medium, MRS L. rhamnosus HN001 showed a higher growth rate compared with YPD and YPF medium. This because of the lack of nutrients in the culture media that in a way limited the growth of these bacteria.
No one of the experimental kinetic studies performed with L. rhamnosus HN001 was observed that bacteria consumed ammonium sulfate; probably due to that bacteria metabolism is inhibited by the organic acids produced, and probably some other metabolites. It is known that glutamine synthetase (glnA) is the main enzyme responsible for the assimilation of ammonium ions in L. rhamnosus, and this enzyme is regulated by the transcription factors GlnR and TnrA.
In response to high concentrations of available nitrogen source in the medium, the transcriptional factor GlnR downregulates the synthesis of glutamine synthetase; also, transcription of the glnA gene is higher when the cells are grown with low nitrogen source [38]. In our case it is probably that during fermentation with L. rhamnosus HN001 has caused an inhibition of the activity of glutamine synthetase by the action of the transcription factor GlnR, in response to high concentration of ammonium sulfate in the culture medium provided.
Throughout these fermentations, L. rhamnosus caused a rapid drop of the media pH due to big amount of lactic acid that it is able to produce; observing at the end of exponential growth phase pH= 4.46 + 0.14. The primary metabolite produced was lactic acid, which was synthetized during both, exponential and stationary growth phases, observing significantly more production when the culture media had glucose as carbon source (P>0.0000) (7.77 and 8.08g/L versus 2.58 and 2.73g/L). Likewise, the concentration of AS in the culture media affected this bacteria’s ability to produce acetic acid (P>0.0000), showing that only produced it when it was cultivated in the media with the lowest AS content (7g/L). Acetic acid was exclusively produced in the stationary growth phase, reaching a maximum of 0.13g/L at the end of the fermentation performed in YPD medium supplemented with 7g/L of AS. Furthermore, Solga (2003) [18] proposed a mechanism which is possible to promote the excretion of ammonia in patients with encephalopathy, which have high concentrations of this toxic metabolite in their system; this mechanism is based on the promotion of the ionization of the ammonium molecule, result that is obtained by acidifying the pH of the intestinal lumen. In this respect if L. rhamnosus HN001 consume glucose as carbon source, is capable of synthesizing a high amount of lactic acid, both in low and high concentrations of ammonia, indicating an increasing in ammonium ionization to ammonia in the intestine.
Instead, it can be said that if L. rhamnosus HN001 consumed fructan as a carbon source, has a lower capacity for synthesis of lactic acid in comparison to glucose consumption; suggesting that L. rhamnosus HN001 can have greater beneficial effect in terms of small intestine rather than colon, mainly in the duodenum where the lactobacillus may dispose of the glucose. However, it is important to emphasize that in all tested media L. rhamnosus HN001 demonstrated survivability, growth and synthesis of organic acids, despite the high concentrations of ammonium in the medium.
The results of the fermentation kinetics of B. lactis Bi-07 in YPD and YPF media supplemented with 7 and
20g/L of AS are shown in Figure 3. In YPD medium B. lactis tended to grow exponentially during 14 to
19h showing a μmax of 0.08h-1; while in YPF media this bacteria presented a shorter exponential growth
phase (~ 8h) and a μmax ranging between 0.11 and 0.14h-1. The type of carbon source had a great impact
on B. lactis growth, noting a faster growth when this bacteria had AF in its culture media (P=0.0001).
In addition, a statistical interaction was found between factors, the ammonium content and the type of
carbon source (P=0.0047), observing that AS supplementation played an important role promoting this
bifidobacteria growth when it had fructan as carbon source (fermentation carried out for 45hours). The GT
was also influenced by these factors observing a lower GT in the fermentation performed in YPF medium
with 20g/L of AS (4.99h-1) (Table 2 and 3).
During these fermentations B. lactis was not able to use completely its carbon source, consuming only 22 to 25% of glucose in the fermentations performed in YPD medium and 14 to 20% of fructans in the ones performed in YPF (Table 2 and 3). In addition, B. lactis presented a qs of 1g/g* h-1 and a yield of 0.06 to 0.09g/g–1 when it was cultivated in YPD medium, as well as a qs of 2g/g* h-1 and a yield of 0.05 to 0.08g/g–1 when it was cultivated in YPF. On the other hand, protein consumption was directly correlated with biomass production, observing approximately 40% of its utilization in the fermentations with greater biomass production. It was not observed ammonium consumption throughout these fermentations.
In these media, the primary metabolites produced by B. lactis were lactic and acetic acids, which were generated in both, exponential and stationary growth phases. Production of lactic acid was greatly influenced by both factors, the AS content (P>0.0000) and the type of carbon source (P>0.0000); observing greater production when media had more ammonium (1.8 versus 2.3g/L, and 3.1 versus 7.1g/L) and fructans as carbon source (1.8 versus 3.1g/L, and 2.3 versus 7.1g/L). Interestingly, a statistical interaction was found between both factors (P>0.0000) noticing that the best conditions for lactic acid production were 20g/L of AS supplementation plus AF, achieving maximum of 7.1g/L of this acid after 72h of fermentation. Furthermore, acetic acid production by B. lactis was also influenced by the type of carbon source (P>0.0000), observing a greater production when this bacteria had glucose in its media (1.1 versus 0.3g/L). Surprisingly it was not detected acetic acid production in fermentations performed in YPF media supplemented with 20g/L of AS.
Regarding the growth of B. lactis Bi-07 in MRS media YPD and YPF, the variable that most influenced biomass production was the carbon source. It is well known the bifidogenic effect exerted by inulin-type fructans in the intestine [39]; however, it has recently been proposed that degradation of the different size fractions of these fructans is through a joint work between different species of Bacteroides genus and various species of Bifidobacterium genus, which exhibit diverse degradative capacity for these molecules [11,39], so these results would not be applicable “in vivo”.
It was observed that the bacteria decrease growth when fermentation was carried out in the YPD medium at an ammonium sulfate concentration of 20g/L, since in this medium there was a maximum biomass, while in YPD medium supplemented with 7g/L of ammonium sulfate produced less amount of biomass. When the growth of B. lactis in YPF medium is compared at both ammonium sulfate concentrations, is observed that the ammonium sulfate production influences biomass production. Moreover, we found that maximum growth was obtained with YPD medium supplemented with 7g/L of ammonium sulfate, using glucose as a carbon source. In contrast, the culture medium where less amount of biomass produced was in the YPF supplemented with 7g/L ammonium sulfate. Surprisingly the same amount of biomass was obtained in the media with 20g/L of ammonium sulfate, with fructans or glucose as a carbon source. It is also important to mention that bacteria growth in MRS medium was greater than in YPD and YPF media; this behavior is due in part to the nutritional content of MRS medium described by Man et al. (1960) [40], which contains glucose, peptone, yeast extract, meat extract, magnesium, manganese, acetate, citrate and polysorbate 80, which facilitate the growth of bifidobacteria.
In this study is possible to consider that in the experimental media YPD and YPF did not obtain similar amount of biomass, due that these media contained less amount of nutrients, in addition, the organic acids produced by microorganisms may have inhibited their growth. In this respect, Desjardins et al. (1990) [41], reported that in fermentations performed with distinct species of Bifidobacterium with pH controlled, bifidobacteria stopped growth inclusive without having consumed completely its carbon source (up to 50% residual). However, in our case, the pH was a limiting, since the required pH for optimum growth of this bacterium oscillates between 6.5-7; and no exists growth to pH value of lesser of 4.0 [42]. Another important issue is that our culture media exclusively contained yeast extract and peptone that is not sufficient stimulus for growing in this strain. Similarly, attending to results found in our experiments, it is possible that concentration of ammonium sulfate present in all experimental culture medium influenced biomass production by the bifidobacteria.
Furthermore, the enzyme β-fructafuranosidasa in B. lactis necessary for the hydrolysis of fructan has an optimum pH to 6 [43] and it has been found in other species this enzyme does not act to pH lower than 4.5 [44]. So it is possible to assume that when medium has low pH, the carbon requirements (glucose and fructose) in bifidobacteria were not maintained free in medium, limiting their growth.
On the other hand, just as L. rhamnosus HN001, there was not consumption of ammonium sulfate in any of the fermentations with B. lactis Bi-07, so that, the enzyme responsible for the assimilation of ammonium, the glutamine synthetase and glutamate dehydrogenase, may have stronger affinity for ammonia than for the ammonium, as in other species of Bifidobacterium is reported activity of these enzymes in the presence of ammonia or hydroxylamine [43]. In the experiments a preference of proteins over the ammonium sulfate in medium was observed in bifidobacteria, assuming that under these conditions, it is difficult to consume ammonium sulfate in order to satisfy their needs for nitrogen. In this regard, it is important to mention that experimental mediums used in this work (YPD and YPF) about 99% of ammonium was in ionized form (NH4+), our results apply primarily to this source of nitrogen in ionized state (NH4+) and not for ammonia (NH3).
Deguchi and co-workers (1985) [44] reported the complete clearance of ammonia in a biological system by the addition of a fermentable substrate and strain of B. bifidum, specifically chosen for its high capacity to incorporate ammonia into their cells. However, these In vivo results obtained, are due to the decrease in pH caused by the release of organic acids produced by the probiotic, promoting ionization of ammonia to ammonium, which is much easier to be excreted In vivo.
Accordingly, it is possible that B. lactis Bi-07 produces more amount of lactic acid when it has fructan as a carbon source and high concentration of ammonium sulfate, thus, it is believed that it might be ideal to promote ionization of ammonia at colon level, main habitat of bifidobacteria [18]. Also, in this studied showed that ammonium sulfate is capable of promoting the growth of bifidobacteria when it consumes fructan as carbon source. About it, we do not know if this is due to change in the metabolism of the microorganism or the incorporation of small amounts of ammonium on bifidobacteria cells. Regarding this result is important to mention the scope of our analysis, since both the chromatographic as colorimetric method, important standard deviations were found; so that a lower consumption of these may not have been detected, due to the sensitivity of the methods [45-48].
Conclusions
This work provides guidelines to analyze the capacity of probiotic bacteria to consume different carbon sources
and ammonium. The In vitro study of fermentations certainly has its limitations regarding extrapolation In
vivo, and never takes into account the complexity of the intestinal microbiota. Despite these limitations, the
studies serve as an important reference for furthering research about bacterial metabolism in people with
hepatic encephalopathy problems. The type of carbon source had a great impact on Bifidobacterium lactis Bi-
07 growth, observing a faster increase when these bacteria consume fructan from agave in the culture media.
From the obtained results, we recommend to consume food with Bifidobacterium lactis Bi-07 and include in
their formulation fructan of agave. The ammonia in the presence of fructan, as carbon source, promotes of
growth B. lactis Bi-07, producing more amount of lactic acid, which helps to further acidification of intestinal
pH, which In vivo would mean increased excretion of ammonia (toxic) in the ammonium form. Although
are evidence the beneficial effects of probiotic consumption in people with HE, there are no criteria to
choose these strains. There is a great variability between strains selected for these studies, which causes a
great diversity in their metabolic activity. Therefore, it is necessary to study in depth the mechanisms that
may be involved in this process for further identification of probiotic strains with more specific metabolic
potential of these diseases.
Acknowledgements
We thank CONACYT and PRODEP for the support provided to carry out this work.
Conflicts of Interests
There is no conflict of interest between the authors. We all agree that the results of this work be published.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!