Biography
Interests
Adeyeye Emmenuel Ilesanmi*, Adesina Adeolu Jonathan & Faleye Francis Jide
Department of Chemistry, Faculty of Science, Ekiti State University, Ado Ekiti, Nigeria
*Correspondence to: Dr. Adeyeye Emmenuel Ilesanmi, Department of Chemistry, Faculty of Science, Ekiti State University, Ado Ekiti, Nigeria.
Copyright © 2018 Dr. Adeyeye Emmenuel Ilesanmi, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Effects of use and re-use of different edible vegetable oils (refined palm olein, soya, olive) on the anti-nutritional factors and antioxidant activities of the plantain chips were investigated using standard analytical methods. For the selected oil (both use and re-use: first and second re-use and the fresh plantain chips (UPC)) had the following result anti-nutritional factors(mg/100g): Tannic acid (1.6-4.18), Total phenol (1.59-4.56), oxalate (1.98-3.60), phytin phosphorus (mg/g)(2.3- 5.80), phytic acid (8.24-19.8), and others (g/100g): alkaloids (0.72-1.56), flavonoids (0.38-1.10) and lectins (0.23-0.65). The Chi-square analysis showed that there were no significant differences among the levels as treated based on the various oils. It was observed that all the anti-nutritional factors level were reduced in all the chips obtained from the re-use oils. The highest antioxidant activities (for all assays: DPPH, ABTS, FRAP, OH inhibition, NO inhibition, flavoniods and total phenolics) were observed in the first (day) frying whereas first and second re-use of the various oils showed reduced antioxidant activities for all the assays investigated. Therefore, for optimum antioxidant activity of fried plantain chips, re-use of oil for frying should be avoided as it leads to reduction in the antioxidant activities.
Abbreviations (if used)
UPC - unprocessed plantain chips; PC1-fresh plantain in each of the oil = day 1 (first frying); PC2 -
fresh plantain fried in oil used in day 1 = second frying; PC3 - fresh plantain fried in oil used in day 2 =
third frying; V - vegetable oil; DPPH - 2,2 -Diphenyl-1-Picryl Hydroxyl; ABTS - 2, 2’-Azino - -Bis--3-
Ethylbenzothiazoline--6-Sulfonic Acid; FRAP - Ferric Reducing Antioxidant Power; OH- hydroxyl; NO
- nitric oxide; SD- standard deviation, CV- coefficient of variation; χ2-Chi-square; S-significantly different,
NS- not significantly different.
Introduction
Plantains and other cooking bananas (Musa spp.) are staple food grown throughout the tropics, they constitute a major source of carbohydrates for millions of people in Africa, the Caribbean, Latin America, Asia and the pacific. Plantain is the common name for herbaceous plants of the genus Musa [1]. The fruit they produce is generally used for cooking in contrast to the soft sweet banana which is sometimes referred to as the desert banana. All members of the genus Musa are indigenous to the tropical regions of south East Asia and Oceania including Malay Archipelago Indonesia, Malaysia, Brunei and the Philippines. Plantains are a major staple food in equatorial Africa and Andean regions. Their attractiveness as food is that they fruit all year round making them a more reliable all season staple food. Steamed, boiled, grilled baked or fried. In Nigeria, plantain is eaten boiled, fried or roasted called “Boli” it is usually eaten with palm oil or groundnut seed. In countries located in Central America and the Caribbean such as Trinidad and Tobago, plantain is simply fried, boiled or added to soup [2]. Plantain (Musa sapientum var. paarasidiaca L.) is a tropical fruit that constitutes a staple food crop in Central and West Africa. Over 2.11 million metric tons of plantains are produced in Nigeria annually which contributes substantially to the nutrition of subtropical local population [3]. In Nigeria, plantain is called “Ogede Agbagba” in Yoruba language; “Ebegehi, Abarika” in Ibo language, “Ayaba” in Hausa language and in Efik language-“Ukom” [4-6]. Plantain chips are the most popular plantain products in Nigeria. They are prepared by slicing the unripe or slightly ripened plantain with a diameter (2mm thick) and fryng in vegetable oil at the temperature between 160-170°c for 10 to 15 minutes.
Usually, only ripe pulp is consumed due to its high sugar content and sensory aspects [7]. However, the green fruit is also consumed in cooking dishes typical of some regions due to its high starch content. In addition, the incorporation of green fruit flour in some products, like biscuits, bread rich in fibres and films, is increasing [7]. The consumption of green bananas (peel and pulp) is beneficial to human health due to the high content of resistant starch, which acts in the body as food fibre [8]. It has been reported that plantain and banana flours can be an important source of polyphenols, compounds considered natural antioxidants [9]. The presence of antioxidant compounds such as phenolic compounds in the human diet is associated with protection effects in the prevention of some chronic- degenerative diseases related to oxidative stress [10] and have proven action on synergistic effects and protective properties against various diseases including cancer, stroke cardiovascular diseases, Parkinson’s and Alzheimer’s diseases [11].
Cooking methods or processing techniques of foods have been reported to create losses of vitamins and other nutrients up to 90% depending on the cooking or processing used [12]. In Nigeria, boiling, roasting and frying are the most commonly used processing methods. Mba et al. [13] reported that low oil temperatures may lead to higher oil uptake unto the fried foods which may in turn affect the nutrients within its matrix.
It is well-known that plants generally contain anti-nutrients which may be acquired from fertilizer and pesticides and several naturally occurring chemicals (secondary metabolites) which are biologically active [14]. They include: saponins, tannins, flavonoids, alkaloids, oxalates, phylates, lectins, etc. These chemicals are capable of eliciting very harmful biological responses, although some of them are widely applied in nutrition and as pharmacologically-active agents [14]. Since their biological effects are diverse and complex, continued investigation and research into their occurrences and effects of processing methods on them become necessary. Functional foods and nutraceuticals are gaining a lot of attention, therefore, it becomes necessary to consider the effects of use and reuse of various vegetable oils on the anti-nutritional and antioxidant capacities of plantain chips fried using three commonly used vegetable oils - refined palm olein, olive and soya bean oils individually and comparatively on repeated frying basis.
Materials and Methods
A bunch of fresh matured unripe plantain was purchased from Iworoko market and certified in the Plant
Science and Biotechnology laboratory of the Ekiti State University, Ado-Ekiti. The vegetable oils (refined
palm olein, olive and soya) used for the frying were purchased from dealers in Ado-Ekiti, Ekiti State and
Akure, Ondo State.
The plantains were peeled to obtain the pulp which was used for the frying. The pulp was thereafter sliced using clean stainless knife (to about 1.5mm thickness) into a clean and dried stainless plate. The sliced plantain was fried in the respective oils using aluminum frying pan (2 litres capacity aluminum) for about 15 minutes until a brown caking appearance was noticed. Fried chips were allowed to cool to room temperature. Cooking gas was used as the heat of frying. The following forms of frying were made: fresh plantain in each of the oil = day 1 (first frying) (PC1), fresh plantain fried in oil used in day 1 = second frying (PC2) and fresh plantain fried in oil used in day 2 = third frying (PC3). 500 ml of each oil was used for the frying. The fried chips were then blended into fine powder with Marlex Excella Mixer Grinder and packed inside clean and dried plastic sample bottles prior to analysis.
4g of the sample was soaked in 100ml 2% HCl for 3 hours and then filtered. 25ml of the filtrate was placed
in a 100ml conical flask and 5ml of 0.03% NH4SCN solution was added as indicator. 50ml of distilled
water was added to give it the proper acidity (pH 4.5). This was titrated with ferric chloride solution which
contained 0.005mg of Fe per ml of FeCl3 used until a brownish yellow colour persisted for 5minutes. Phytin
phosphorus (Pp) was determined as follows:
Each milligram of iron is equivalent to 1.19mg of Pp.
The phytic acid content was then calculated by multiplying with value of Pp by 3.5
200mg of the sample was weighed into a 50ml bottle. 10ml of 70% aqueous acetone was added and properly
covered. The bottles were put in an orbital shaker and shaken for 2 hours at 30°C. Each solution was then
centrifuged and the supernatant stored in ice. 0.2ml of each solution was pipetted into test tubes and 0.8ml
of distilled water was added. Standard tannic acid solutions were prepared from 0.50 mg/ml stock and the
solution made up to 100ml with distilled water.
0.5ml Folin reagent was then added to both sample and standard followed by 2.5ml of 20% Na2CO3. The solutions were then vortexed and allowed to incubate for 40 minutes at room temperature after which absorbance at 725nm was red against a reagent blank concentration of the sample from a standard tannic acid curve [17].
1g of the sample was weighed into 100 ml conical flask. 75ml of 0.75 M H2SO4 was added and the solution
was carefully stirred intermittently with a magnetic stirrer for about 1 hour and then filtered using Whatman
filter paper. 25ml of sample filtrate was collected and titrated hot (80-90°C) against 0.1M KMnO4 solution
to the point when a faint pink colour appeared that persisted for at least 30 seconds [18]. Oxalate was
calculated as follows:
Alkaloid determination was carried out following the procedure of Harborne [19]. 5.0g of the sample was
weighed into a 250ml beaker and 200ml of 10% acetic acid in ethanol was added and covered and allowed to
stand for 4h. This was filtered and the extract was concentrated on a water bath to one quarter of the original
volume. Concentrated ammonium hydroxide was added drop wise to the extract until the precipitation
was complete. The whole solution was filtered and the precipitate was collected after washed with dilute
ammonium hydroxide. The residue is the alkaloid which was dried and weighed.
The method of Boham and Kocipai-Abyazan [20] was followed in the determination of flavonoid. 5g of
the sample was extracted three times with 100ml of 80% aqueous methanol at room temperature. The
whole solution (125ml) was filtered through Whatman filter paper. The filtrate was later transferred into an
evaporating dish and evaporated into dryness and weighed to a constant weight.
The total phenolic content of the various plantain chip flour sample was determined by the Folin-Ciocalteu assay as described by Waterman and Mole [21]. 500μL of Folin reagent was added and mixed with a solution containing 100μL of the extract and 2ml of distilled water. 1.5mL of 7.5% sodium carbonate was then added to the solution and the volume was made up to 10mL with distilled water. The mixture was left to stand for 2 hours after addition of the sodium carbonate for which the absorbance of the mixture was measured at 760nm using a Lambda EZ150 spectrophotometer (Perkin Elmer, USA). The standard used was tannic acid. Tannic acid content of samples was determined in triplicates and the results expressed as mg tannic acid equivalents per gram of the sample.
The total flavonoid content of the plantain chip flour sample was determined using a slightly modified method reported by Meda et al. [22]. 0.5mL of the tea infusion was mixed with 0.5mL methanol, 50μL of 10% AlCl3, 50μL of 1mol L-1 potassium acetate and 1.4mL water, and allowed to incubate at room temperature for 30 min. Thereafter, the absorbance of each reaction mixture was subsequently measured at 415nm. The total flavonoid was calculated using quercetin as standard by making use of a seven point standard curve (0-100μg/ml). The total flavonoid content of samples was determined in triplicates and the results were expressed as mg quercetin equivalent per gram of the sample.
The reducing power of the extracts was determined by assessing the ability of each extract to reduce FeCl3 solution as described by Oyaizu [23]. 1ml of the infusion was mixed with 1mL 200mM sodium phosphate buffer (pH 6.6) and 1mL 1% potassium ferricyanide. The mixture was incubated at 50°C for 20 min and then 1mL 10% trichloroacetic acid (TCA) was added. This mixture was centrifuged at 650 x g for 10 min. 2mL of the supernatant was mixed with an equal volume of water and 0.4mL of 0.1% ferric chloride. The absorbance was measured at 700nm. The ferric reducing antioxidant power was expressed as mg ascorbic acid equivalent/g of the sample.
In vitro DPPH radical scavenging activity was done according to the method of Williams et al. [24], with some modifications. The stock solution was prepared by dissolving 24 mg DPPH with 100mL methanol and then stored at -4°C until needed. The working DPPH solution was obtained by mixing 10mL stock solution with 50mL methanol to obtain an absorbance of 1.1 units at 515nm using the spectrophotometer. 0.5mL of the extract was diluted with 2mL of methanol to obtain a mother solution. 450μL of the mother solution was allowed to react with 2550μL of the DPPH working solution for 1 hour in the dark. Then the absorbance was taken at 515nm. The scavenging activity was estimated based on the percentage of DPPH radical scavenged.
ABTS radical scavenging activity of the plantain chip flour sample was determined using ABTS antiradical assay as described by Awika et al. [25]. The ABTS•+ (mother solution) was prepared by mixing equal volumes of 8mM ABTS and 3mM potassium persulphate (K2S2O8) (both prepared using deionized water) in a volumetric flask, which was wrapped with aluminum foil and allowed to react for a minimum of 12 hours in a dark place. The working solution was prepared by mixing 5ml of the mother solution with 145ml phosphate buffer (pH 7.4). A range of trolox (6-hydroxy-2, 5, 7, 8-tetramethylchroman-carboxylic acid) standard solutions (100-1000μM) were prepared in acidified methanol. The working solution (2.9ml) was added to the Trolox standard (0.1ml) in a test tube and mixed with a vortex. The test tubes were allowed to stand for exactly 30 min. The absorbance of the standards and samples were measured at 734nm with a Lambda EZ150 spectrophotometer. The result was expressed as μmolTrolox equivalents/g sample, on dry weight basis.
The ability of the plantain chip flour to prevent Fe2+/H2O2 induced decomposition of deoxyribose was carried out using the method of Halliwell and Gutteridge [26]. Freshly prepared extract (0-100μL) was added to a reaction mixture containing 120μl of 20mM deoxyribose, 400μl 0.1M phosphate buffer pH 7.4, 40μL 20mM hydrogen peroxide and 40μL 500mM FeSO4, and the volume was made to 800μL with distilled water. The reaction mixture was incubated at 37˚C for 30min and then stopped by the addition of 0.5mL of 2.8% TCA. This was followed by the addition of 0.4ml of 0.6% TBA solution. The reaction tubes were subsequently incubated in boiling water for 20min. The absorbance was then measured at 532nm with a spectrophotometer.
The nitric oxide radical scavenging capacity of the plantain chip flour was measured by Griess reaction [27]. Sodium nitroprusside (2.7mL, 10mM) in phosphate buffered saline (PBS) was added to 0.3mL of the plantain chip flour sample and incubated at 25°C for 150 min. 0.5ml of the incubated aliquot was added to 0.5mL of Griess reagent: 1% (w/v) sulfanilamide, 2% (v/v) orthophosphoric acid and 0.1% (w/v) naphthylethylenediamine hydrochloride, (prepared in amber bottle and keep away from light). The absorbance was measured at 546nm. Percentage of inhibition of the nitric oxide generation is measured by comparing the absorbance values of control and samples.
Results
The levels of anti-nutritional factors in the plantain chips (unprocessed and fried) at 24hours interval for
three days are depicted in Table 1. The results for the unprocessed (UPC) and fried plantain chips are in the
following ranges: tannic acid (mg/100g)(UPC: 4.18; VPC 1,2,3: 2.00-2.09; SPC1,2,3: 1.60-2.73; OPC1,2,3:
2.10-2.60), total phenol (mg/100g)(UPC:4.56; VPC1,2,3: 1.70-2.43; SPC1,2,3: 1.59-2.69; OPC1,2,3:
1.67-2.36), oxalate (mg/g)(UPC: 2.61; VPC1,2,3: 2.52-2.61; SPC1,2,3:2.16-3.15; OPC1,2,3: 1.98-3.60),
phytin-phosphorous (mg/g) (UPC: 5.34; VPC1,2,3: 2.55-3.48; SPC1,2,3: 2.32-3.94; OPC1,2,3: 3.02-5.80),
phytic acid (mg/g)(UPC: 18.9; VPC1,2,3: 9.06-12.4; SPC1,2,3:8.24-14.0; OPC1,2,3: 10.7-20.6), alkalods
(g/100g)(UPC:1.56; VPC1,2,3: 1.25-1.36; SPC1,2,3: 0.72-1.88; OPC1,2,3:1.30-1.37), flavonoids(g/100g)
(UPC:1.10: VPC1,2,3: 0.38-1.06; SPC1,2,3: 0.41-1.01; OPC1,2,3: 1.00-1.06) and lectins (g/100g) (UPC:
0.20; VPC1,2,3: 0.40-0.42; SPC1,2,3: 0.23-0.40; OPC1,2,3: 0.42-0.65).
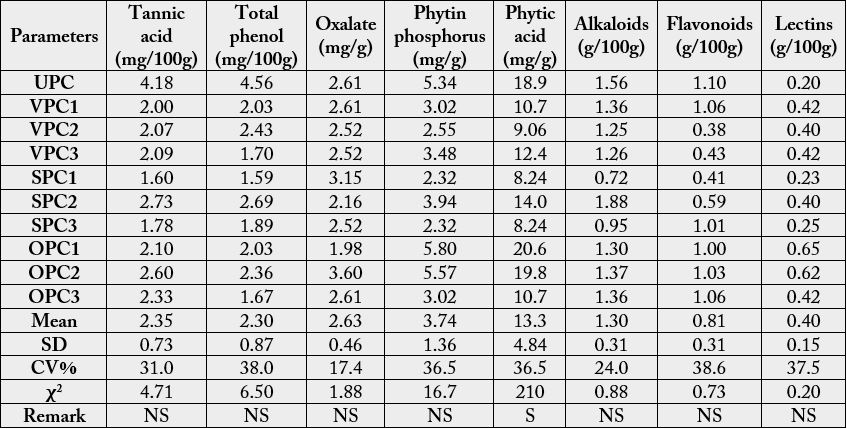
U= unprocessed, PC= plantain chips, 1= product of first frying (first day), 2= product of second frying (first re-use of oil), 3= product of third frying (second re-use of oil), SD= standard deviation, CV= coefficient of variation, χ2=Chisquare at n-1, r=0.05, S=significantly different, NS= not significantly different
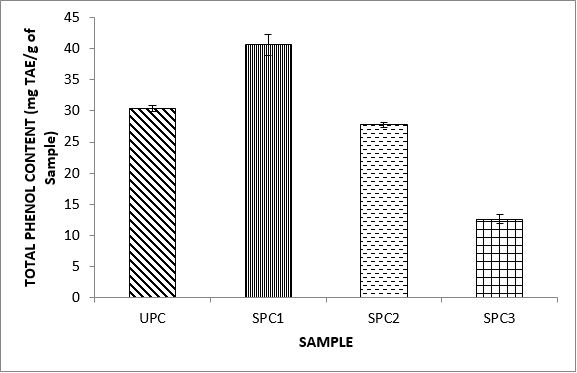
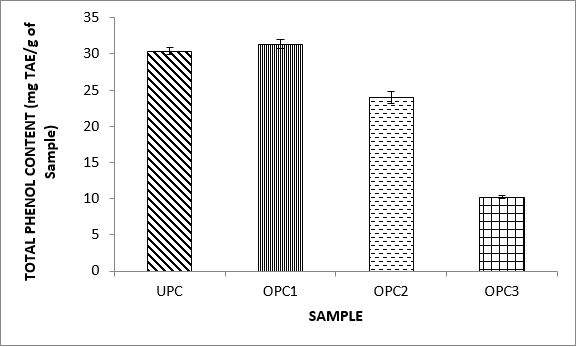
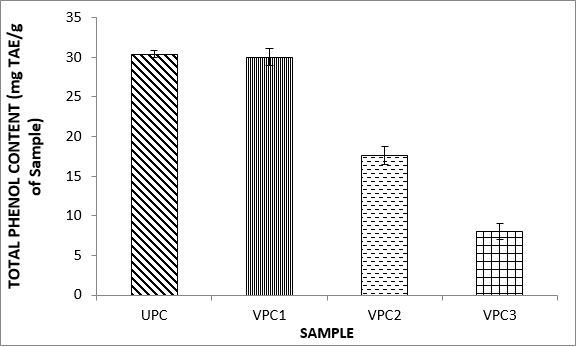
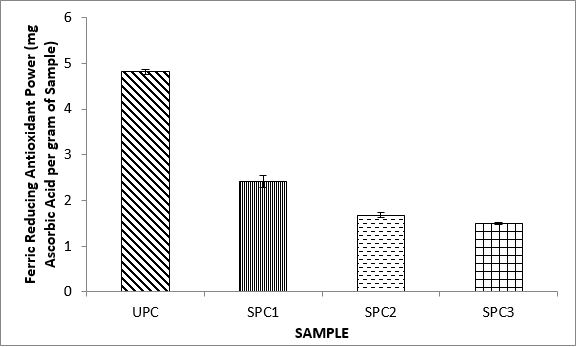
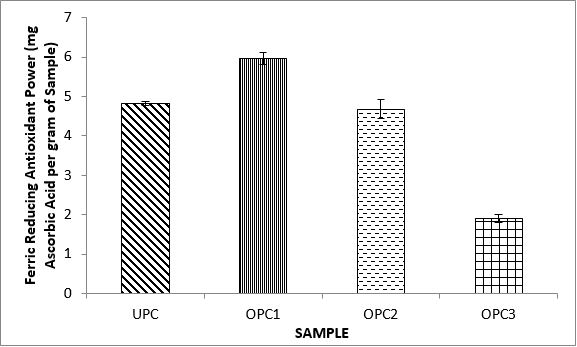
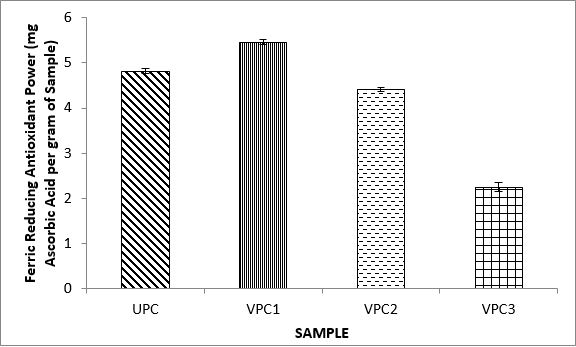
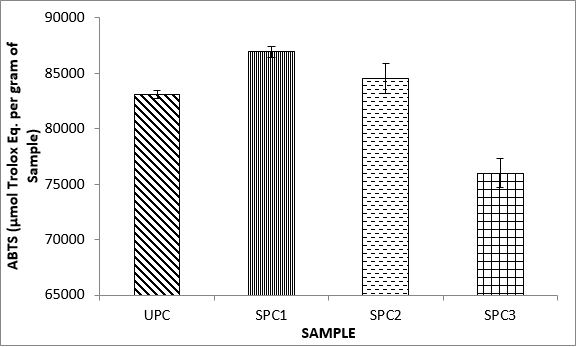
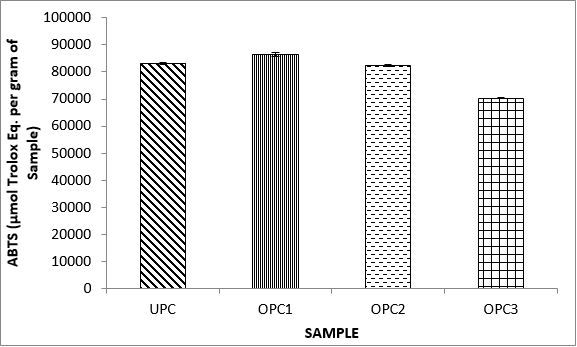
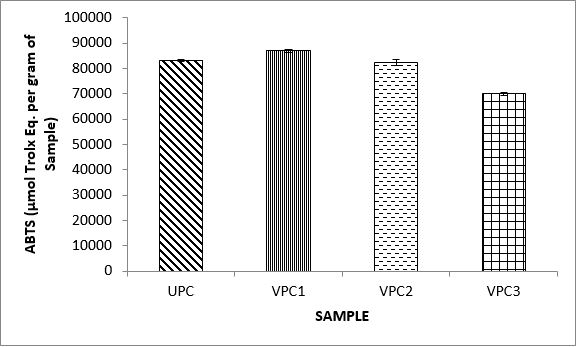
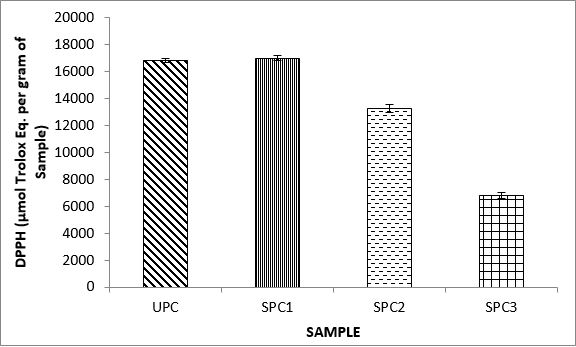
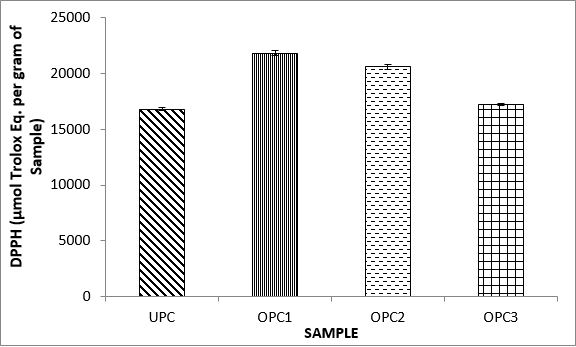
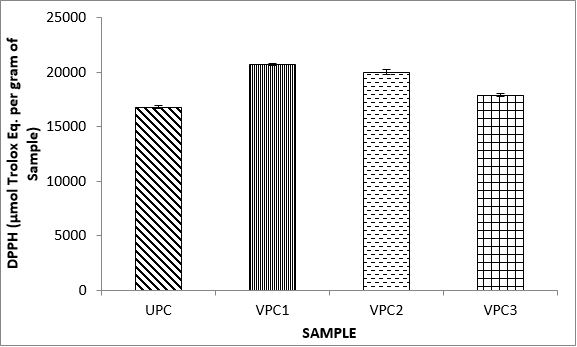
Variations in the antioxidant properties of the unprocessed and processed plantain chips using different oils are depicted in Figures 1-21. Variations in the total flavonoids content of the unprocessed and fried plantain chips using soya bean oil, olive oil and refined oil were shown in Figures 1-3. The highest enhancement in the levels as shown in the figures were SPC1, OPC1 and VPC1. This suggests that frying plantain chips only once will enhance the level of flavonoids in the product better than the repeated oil frying. Similar trends were observed in the results for total phenol (mg TAE/g) (Figures 4-6); ferric reducing antioxidant power (FRAP) (mg Ascorbic acid/g) (Figures 7-9); ABTS (µmolTroloxEq/g) (Figures 10-12); DPPH (µmolTroloxEq/g) (Figures 13-15); % NO inhibition (Figures 16-18); and % OH inhibition (Figures 19- 21).
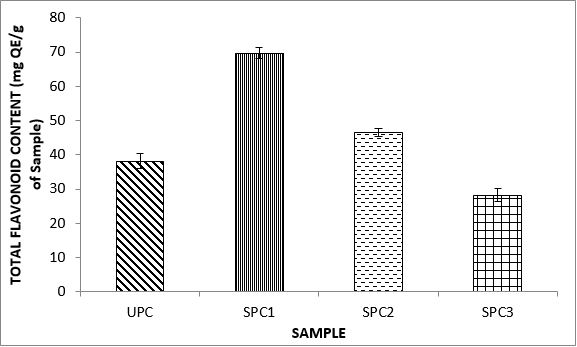
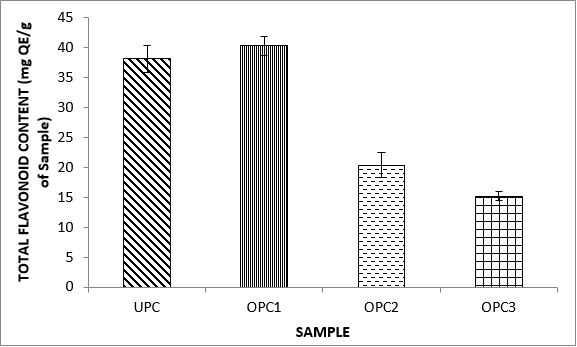
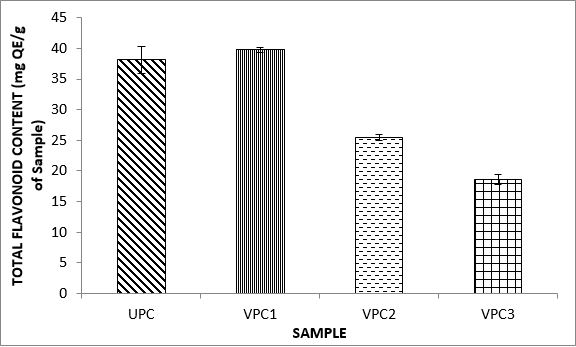
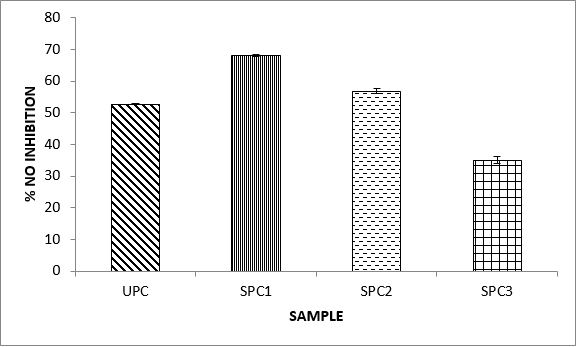
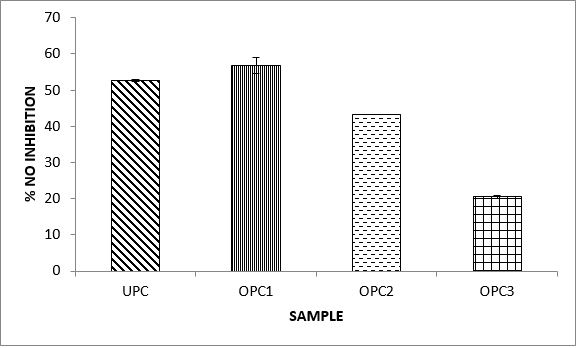
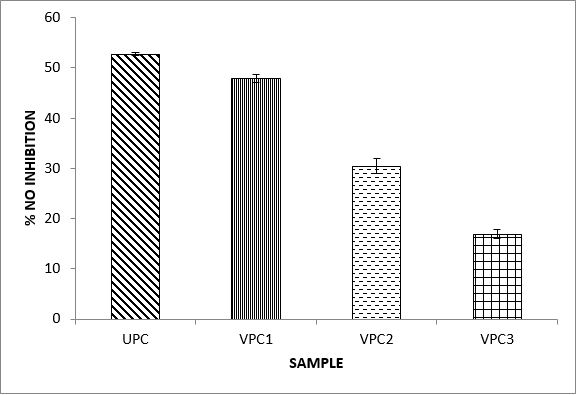
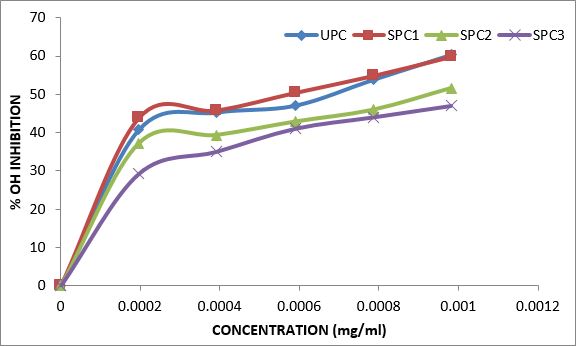
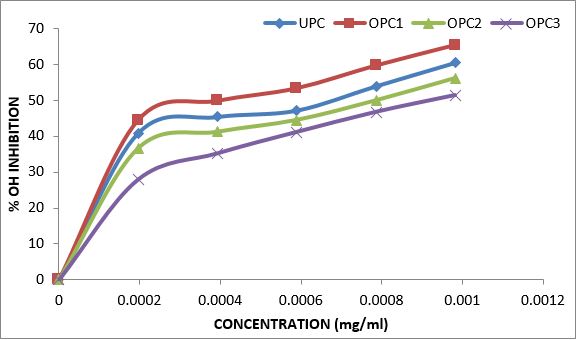
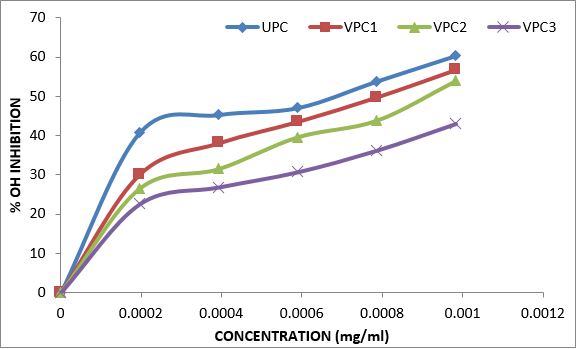
Discussions
Vegetables tannin/tannic acids are water soluble polyphenolic compounds that are widely distributed in the
plant kingdom. Tannin can produce undesirable effects in food when present in considerably high amounts
[28]. They have ability to form a complex with proteins through hydrogen bonding and covalent linkages.
This results in the precipitation of proteins to make it unavailable for absorption [28,29]. Levels of tannic
acid in the present report for both processed (fried) and unprocessed plantain chips were comparably
lower (except UPC, 4.18mg/100g) than the levels reported for unripe, the ripe and the overripe plantain
peels (2.84-5.39mg/100g) [6] but higher comparatively to the values observed in the unripe plantain flour
(1.58%) [30]. Tannic acid/tannins cause decreased feed consumption in animals and also caused decreased
palatability and reduced growth rate [29,31]. Incidence of esophagus cancer has also been linked with dietary
tannins [28]. Tannic acid, when fed to different animal species, has been shown not only to affect the
digestibility and absorption of nutrients but also to affect different internal organs. Chang and Fuller [32]
observed fatty livers in chicks fed diets containing tannic acid. Karim et al. [33] reported necrosis of the liver
and kidneys of chicks fed diets containing 1-3% tannic acid. Also varying degrees of desquamation in the
surface epithelium and necrosis of the epithelial layer of the small intestine were found in some birds. Tannic
acid when injected into rats caused disaggregation of liver polyribosomes, altered microsomal enzyme
activity and inhibited nucleic acid and protein synthesis at the cellular level [34]. According to Mitjavila et
al. [34] however, feeding 3.2% tannic acid to rats for six months, did not find effects on liver function, triglyceride
concentration or oxidative enzyme content, although growth was retarded. If tannin concentration
in the diet becomes too high, microbial enzyme activities including cellulose and intestinal digestion may be
depressed [35]. Tannins also form insoluble complexes with proteins and the tannin-protein complexes may
be responsible for the antinutritional effects of tannin containing foods [36].
In literature reports, higher contents of phenolic compounds have been reported in fruit peels than in the pulp. Notable examples are in banana [37]; mango [38], tomato [39] and in Ternnha and marmelo banana cultivars (33.3 and 31.6mg/GAE and 61.0 & 36.3mg/GAE) respectively [7].
The level of total phenol in the present sample (fried and unprocessed) were comparably lower than what was reported for unripe plantain flour (1.42%) [30]. Phenolic compounds tend to accumulate in dermal tissues in various plant parts (leaves and fruits), due to the important role they play in protecting against ultraviolet radiation, acting as attractant in the dispersal of fruits and as defense substance against pathogens, predators and insects [30].
Oxalic acid (C2O4H2) in combination with its salts or minerals form oxalates. Oxalic acid is present in the cell sap of many green leafy vegetables. Depending on plant species, oxalates can occur as insoluble salts of calcium, magnesium and iron and soluble salts of potassium and sodium or as a combination of these two forms [28]. The distribution of oxalic acid had been reported to be uneven in plant and varies among species. Generally, the amount of oxalate is high in leaves followed by seeds and less in stems, hence, consumption of leafy vegetables has more of a concern where there is a risk of high oxalic acid concentration [40]. In the present report, the levels of oxalate (mg/100g) are comparably higher than those reported for bitter leaf (1.00µg/g), cassava leaf (1.05µg/g), Anacadium occidentalis leaf (1.60µg/g), water leaf (0.92µg/g) [41] but comparatively lower than the values reported for ripe and unripe plantain fruits (8.3- 10.2mg/g) [42]. Oxalate could be toxic when consumed in an unprocessed food [43]. Too much of soluble oxalate in the body prevents the absorption of soluble calcium ions as the oxalate binds the calcium ions to form insoluble calcium oxalate complexes. As a result of this, people with tendency to form kidney stones are advised to avoid oxalate-rich foods [44].
The nutritional value of phytin phosphorus varies with the type of compound to which it is bound. Because of these differences in working with plant materials especially fruits, grains and seeds, an accurate method is needed for the determination of the peculiar type of phytin phosphorus which prevails to the extent of 70 to 90% in grains and seeds. In nature, phytin phosphorus, presumably occurs as sodium and magnesium salts of inositol hexaphosphoric acid [45]. The levels of phytin phosphorus in the fried and unprocessed plantain chips (mg/g) (UPC-5.34; processed -2.32-5.80) were comparably higher than what was observed in the raw and processed Treculia africana seeds flour [46]. Any phosphorus value that is present in phytin or phytic acid will be chelated by the phytin, hence, such phosphorus will not be biologically available. So the higher the value of phytin phosphorus the less the biological availability of such phosphorus.
Phytic acid (myo-inositol hexaphosphoric acid) is a natural substance that acts as a major storage of phosphorous in all leafy vegetables. It chelates essential mineral nutrients in human body making them less available for consumption [47]. Its levels in the present report (processed and unprocessed chips) were comparably higher than those reported for common vegetables in Nigeria (5.80-8.11µg/g) [41] and compared favourably with the values reported for Treculia africana seeds (processed and unprocessed) flours (5.78-14.1 mg/100g) [46]. Besides lowering the bioavailability of minerals and inhibiting the digestibility of proteins, phytic acid is also implicated in the “hard-to-cook” phenomenon of legumes [48]. Despite its antinutritive effects, phytic acid is also known to demonstrate a wide range of beneficial effects to health including in vitro antioxidant that inhibits Fenton reactions leading to lipid peroxidation [49] under certain conditions, such as high pH and presence of iron; and anti-carcinogenic effects, decrease in plasma cholesterol and triglycerides [50-52], lowering toxicity of heavy toxic metals by reducing their bioavailability [53].
Levels of alkaloids, flavonoids and lectins observed in the processed and unprocessed plantain chips were generally low (0.20-1.88). Similar observations have been made by Eleazu et al. [30] for alkaloids and flavonoids in unripe plantain flour (0.981-1.31%) and by Adesina and Adeyeye, [46] in Treculia africana seeds flour (both processed and unprocessed). Alkaloids have been reported to cause gastrointestinal and neurological disorders [54]. Alkaloids are considered to be anti-nutrients because of their action on the nervous system, disrupting or inappropriately augmenting electrochemical transmission. For instance, consumption of high tropane alkaloids will cause rapid heartbeat, paralysis and in fatal case, lead to death. Uptake of high dose of tryptamine alkaloids will lead to staggering gate and death. Indeed, the physiological effects of alkaloids have on humans are very evident. Cholinesterase is greatly inhibited by glycoalkaloids, which also cause symptoms of neurological disorder. Other toxic action includes disruption of the cell membrane in the gastrointestinal tract [55].
Lectins are carbohydrate binding (glycol) proteins which are ubiquitous in nature. In plants they are distributed in various families and hence ingested daily in appreciable amounts by both humans and animals. One of the most important nutritional features of plant lectins is their ability to survive digestion by the gastrointestinal tract of consumers. This allows the lectins to bind to membrane glycosyl group of the cells lining the digestive tract. This interaction triggers harmful reactions which make it anti-nutritional or toxic as they can disrupt lipid carbohydrate and protein metabolism [56].
Statistical analysis (Chi-square) showed that the effects of use and re-use of the various oils on the antinutritional factors were not significantly different except for phytic acid. The re-use of the various oils followed similar trends in their effects on the antinutrient contents of the fresh and fried products.
The difference (as well as the percentage difference values) in the anti-nutritional factors level between unprocessed and processed plantain chips are presented in Table 2. The results showed that there were reductions in the levels of anti-nutritional factors with respect to the unprocessed plantain flour (UPC/ VPC1, UPC/VPC2 and UPC/VPC3) across all the anti-nutrients considered except lectins which showed significant increment.
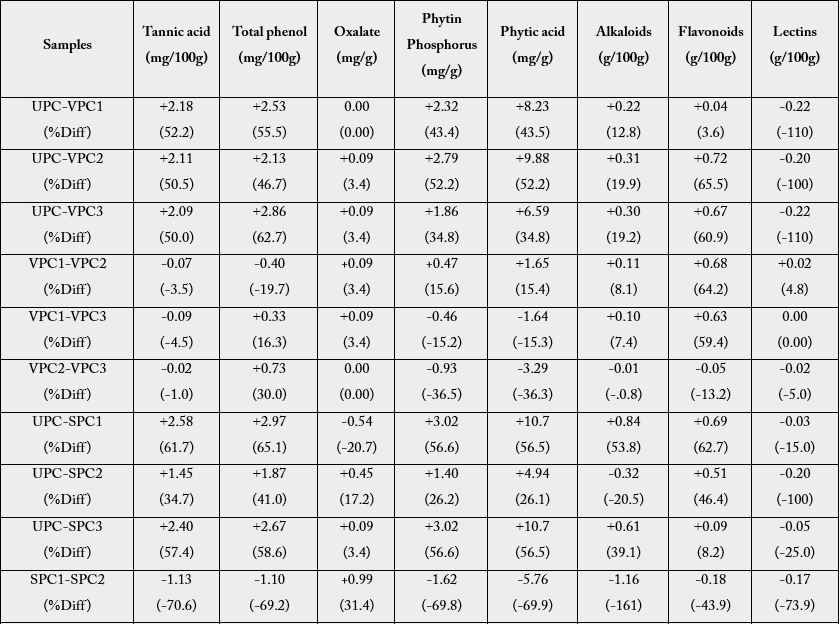
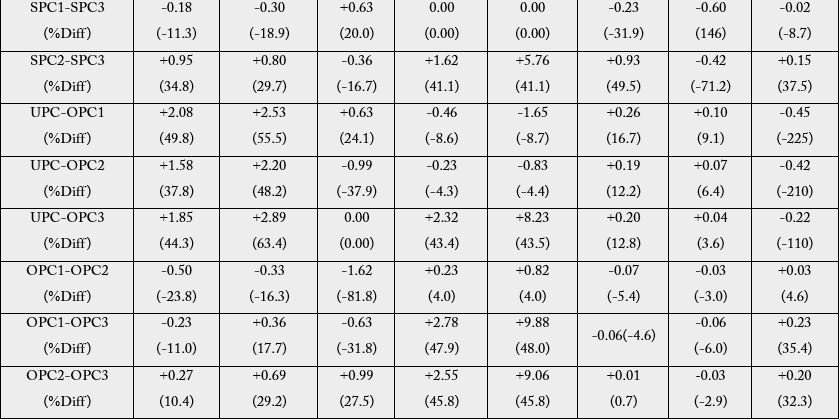
% Diff= percentage difference, + = enhancement in the level of anti-nutritional factor of the reference, - = decrease in the level of anti-nutritional factor of the reference.
Enhancement of tannic acid and total phenol were observed in VPC2 (second day frying whereas oxalic, phytin phosphorous, phytic acid, alkaloids, flavonoids and lectins were observed to be reduced. Tannic acid, phytin phosphorous and phytic acid were enhanced in the product obtained from the third day frying (VPC2 - VPC3 and VPC1 - VPC3) with % enhancement range of 3.29 - 36.5. The differences between UPC and SPC1, SPC2 and SPC3 were positive for tannic acid, total phenol, phytin phosphorus and flavonoids with % difference range of 3.40 - 65.1. Frying with olive oil (UPC/OPC1, OPC2 and OPC3) also showed similar trend with respect to reduction and enhancement of the levels of anti-nutritional factors. Looking at each oil used for the frying, the highest reduction respectively for individual anti-nutrients is as follows: tannic acid: 52.2% (VPC1), 61.7% (SPC1) and 49.8% (OPC1), total phenol:62.7% (VPC3), 65.1% (SPC1) and 63.4% (OPC3); oxalate-3.4% (VPC2 and VPC3), and 31.4% (SPC2) and 27.5% (OPC3); phytin phosphorus - 52.2% (VPC2), 56.6% (SPC1) and 47.9% (OPC3); phyticacid-52.2% (VPC2), 56.5% (SPC1) and 48.0% (OPC3); alkaloids: 19.9% (VPC2), 53.8% (SPC1) and 16.7% (OPC1); flavonoids: 65.5% (VPC2), 62.7% (SPC1) and 9.1% (OPC1) and lectins: 4.8% (VPC1/VPC2), 37.5% (SPC2/SPC3) and 35.4% (OPC2/ OPC3). However, with reference to the unprocessed vis-a-vis various products from oil used, the first day fried chips recorded the highest levels of reduction of the ant-nutritional factors.
In the literatures several authors have reported the effects of different heat treatments on anti-nutritional factors. For instance, Osman [57] observed significant reduction in the values of trypsin inhibition activities, phytic acid by roasting and autoclaving of black bean, faba bean and dry bean. Vijakumari et al. [58] reported that cooking or autoclaving of Dolicos lablab seeds reduced the tannin contents by 70 and 60% respectively.
The treatment involves steam/heat treatment for a predetermined time at a predetermined temperature and due to the treatments the anti-nutritional factors are at least partially broken down and determined constituents such as fats and minerals become better accessible thereby causing the nutritional values of the food to increase.
The increase in the antioxidant parameters during the first frying operation for all the chips in the various oils considered may be due to attractions in chemical structures/composition, causing them to be more readily available and detected in the supernatants of the extract [59]. Similar observations have been made by Oladele and Santosh [60] in which an increase in soluble polyphenols contents of boiled unripe plantain in non-extractable polyphenols in the grilled sample of plantains. Generally the frying process resulted in different types of bonds being formed between phytochemicals present and cell structure which subsequently resulted in higher or lower cleavage of phenolic bonds according to the type of heat applied, resulting in the different responses observed in the present study.
A similar observation to that made in this study was reported by Makris and Rossiter [61] in a study involving flavonols where baking resulted in a 7-25% gain in quarcetin while boiling led to an increase of 18% in quarcetin concentration. It is also possible that higher content of hydrolysable polyphenols in almost all the processed samples than raw samples may be due to interactions between polysaccharides and pholyphenols. One of the mechanisms proposed to explain the interactions that occur between polysaccharides and polyphynols suggests the formation of hydrogen bonds between the hydroxyl groups on the polyphenols and the oxygen atoms from the sugar of the cell wall polysaccharides: this interaction can then form dextron gels, which are able to encapsulate the polyphenols [62].
The influence of home cooking methods, such as frying on the antioxidant activity of vegetables by Jimenez- Monreal et al. [63] showed that garlic loss higher than 50%, asparagus between 30%-40%, Swiss chord, cauliflower and pepper between 5% and 30% considering ABTS radical scavenging capacity. Depending on the amount of water that the frying process removed from the food and that is mixed with triacylglycerols (TAG), these compounds may be hydrolysed and form free fatty acids, monoacylglycerols and diacylglycerols and subsequent oxidation could result in the decreased antioxidant characteristics observed in the second and third frying process [64].
The ABTS radical scavenging assay revealed that antioxidants capacities of fried plantain chips from various oils used had the same trends as the DPPH radical scavenging assay. The first frying process increased antioxidant capacities compared with the unprocessed, second and third frying process. These results are consistent with the work of Dewanto et al. [65], Turkman et al. [66] and Francisco and Resurreccion [67] who reported that heat treatments enhanced antioxidant capacity in pepper, green bean, broccoli, spinach, sweet corn and pea nut. Many antioxidant compounds in plants are mainly present as covalently bound forms within soluble polymers [68]. It is possible that heat disrupts the cell wall and releases antioxidant compounds, leading to an increase in antioxidant capacity mainly for the first frying process.
Several flavonoids reduce the cellular lesions related to ischaemia, by interfering with the activity of nitric oxide (NO) synthesis. The nitric oxide is produced by various types of cells such as the endothelium cells and the macrophages. The nitric oxide (NO) is produced through the constitutive activity of nitric oxide synthesis. It plays a role for the maintenance of the dialation of the blood-vessels, relaxation of the smooth muscles, the signal of transduction and inflammation [69]. However, at high concentration, it induces an irreversible oxidative damage on cellular walls [70].
Conclusions
The study showed that the unprocessed and processed (fried) plantain chips [from various oils (use and
re-use: first, second and third frying)] had low levels of anti-nutritional factors and appreciable levels of
antioxidant activities that could support good health based on the seven different assays used. It was observed
that the products from re-used oils had reduced anti-nutritional factors. Also from the antioxidant assays
(DPPH, ABTS, FRAP, OH, NO, flavonoid and total phenolic) as compared with the unprocessed plantain,
first (day) fried products had the highest level of antioxidant activities whereas the products from first and
second re-used oils showed reduced antioxidant activities. The general trend of the antioxidant activities
among the plantain chips obtained from the various oils goes thus: first fried product > first re-used oil
product > second re-used oil product. For optimum antioxidant activity of fried plantain chips, re-use of oil
for frying should be avoided as it leads to reduction in the antioxidant activities.
Conflict of Interests
The authors declare no conflict of interest.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!