Biography
Interests
Suleiman, M.* & Abdullahi, B. K.
Department of Biology, Umaru Musa Yar’adua University, Katsina, Nigeria
*Correspondence to: Suleiman, M., Department of Biology, Umaru Musa Yar’adua University, Katsina, Nigeria.
Copyright © 2018 Suleiman, M. & Abdullahi, B. K. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Quality deterioration in sorghum grain caused by S. zeamais after six months post treatment with some botanicals was determined by proximate and mineral analyses of the grain before and after infestation. Twenty grams of sorghum were treated with leaf powders of Euphorbia balsamifera Aiton, Lawsonia inermis L., Mitracarpus hirtus (L.) DC and Senna obtusifolia L. at the concentrations of 2.5 x 104, 5.0 x 104 and 10.0 x 104ppm along with conventional insecticide (permethrin) at 0.056 x 104ppm. The results showed that moisture content was not significantly different (p > 0.05). Ash was higher in treated grains than in untreated ones and vice versa for crude fibre. Percent crude lipid in treated grains ranged from 6.50 ± 0.00 to 7.17 ± 0.17% and increased with increased in concentrations. The amount of crude protein decreased drastically in untreated samples compared to treated grains where it varied between 8.75 ± 0.01 to 8.99 ± 0.41%. Carbohydrate content in the sorghum grains was significantly different (p < 0.05) among the botanical treatments. There was a substantial decrease in the minerals content in infested grains. Findings of this study have revealed that all the botanical powers have potentials of reducing effect of S. zeamais on the nutritional value of sorghum grains with E. balsamifera as the most effective.
Introduction
The role of agriculture remains significant in the Nigeria economy despite the strategic importance of the oil sector. Agriculture provides primary means of employment for Nigeria and accounts for more than one third of total gross domestic product (GDP) and labour force [1]. More than 70% of the working adult populations in Nigeria are employed in the agricultural sector directly or indirectly and over 90% of Nigeria’s agricultural output comes from peasant farmers who dwell in the rural areas where 60% of the population live [1]. The major cereal crops in Nigeria are rice, maize, sorghum, wheat, and millet. Sorghum is the staple food crop that is economical to grow in dry areas of many regions of Africa where the climatic conditions are unfavourable for the growth of other crops [2].
Sorghum is produced for human nutrition and animal feeds all over the world, and over half (55%) of it is used for human consumption in Asia and Africa [3]. It is the major crop for many poor farmers, especially in Africa, Central America and South Asia [4]. Grain sorghum is used for flours, porridges and side dishes, malted and distilled beverages as well as popped grain. It is the main source of calories and protein in some regions of Africa and Asia [5]. Sorghum is the primary food crop in virtually all parts of northern Nigeria [6].
FAO (2006) reported processed sorghum grains and flour as important sources of calories and proteins to the vast majority of the population as well as for poultry and livestock [7]. Sorghum grain flour has an average energy value of 356kcal/100g and protein content which ranges from 7 to 15% [8,9].
Despite its nutritional value, sorghum production has been hindered by biotic and abiotic constraints. Among the biotic constraints, insect pests are the major devastating factors attacking the grains during storage [10]. Once infestation is established, insect pests cause gradual and progressive damage leading to losses in weight, nutritional, organoleptic and aesthetic quality of grains [11]. Goftishu and Belete (2014) emphasized that S. zeamais damage leads to quantitative and qualitative deterioration of sorghum grain [12]. These losses could be influenced by the storage time and population of the insects involved in the infestation. The qualitative loss is attributed to change in biochemical components such as carbohydrates, fats and proteins [13]. Damage to sorghum grains in storage in northern Nigeria due to this pest ranges between 4 and 10% [14].
Qualitative loss arises primarily from the alteration of the physical appearance and chemical constituents of the grains with insect frass and debris that could lead to detectable reduction in important nutrients such as sugar, proteins, fats, minerals and vitamins [15]. In order to reduce these losses, some botanicals of various forms have been tested by some researchers against the weevils in maize grains [13].
However, there is limited information on effects of botanicals on nutritional quality of sorghum grains infested by S. zeamais or any other insect pest. Considering the economic importance of sorghum and the damage caused to it by S. zeamais, the present study was aimed at assessing the qualitative loss in the infested sorghum grains treated with different botanical powders and to determine the effectiveness of the botanicals in protecting the grain quality.
Materials and Methods
Twenty grams of sorghum grains of the variety “Farar Kaura” in the plastic bottles was treated with 2.5, 5.0
and 10.0 x 104ppm of E. balsamifera, L. inermis, M. hirtus and S. obtusifolia along with 0.056 x 104ppm
of permethrin powder. Another 20g of the grains was placed in separate bottle without any treatment.
All treatments were arranged in a completely randomised design (CRD) with 3 replicates. Ten adults of
S. zeamais were introduced into the bottles and allowed them to stay for 14 days in an incubator at 30°C
and 70% R.H. after which they were removed. The grains were maintained in the same condition in the
incubator for 6 months and then sieved to remove the powders and any other unwanted particles. The sieved
samples were then washed with deionised water and ovum dried at 65°CC for 6 hrs [16]. The dried samples
were ground into fine powders using laboratory stainless steel mortar and pestle. Clean sorghum grains were
earlier obtained and ground into fine powder before infestation (as untreated un-infested). The powders
were placed in well-labelled bottles separately and kept in the laboratory for proximate and mineral analyses.
To assess the qualitative losses caused to sorghum grains by S. zeamais, the ground samples were analyzed
for moisture, ash, crude fibre, crude lipid, crude protein and carbohydrate based on the recommendation of
the Association of Official Analytical Chemists [17,18]. All analyses were conducted in triplicates.
Five grams of the sample powders were weighed into pre-weighed crucible (W1) and placed into a drying
oven at 105°C for 24hrs. The crucible was removed, cooled in a desiccator and re-weighed. The processes
of drying, cooling and re-weighing were repeated until a constant weight (W2) was obtained. The moisture
content was determined as:
Where:
W1 = Weight (g) of sample
W2 = Constant weight (g) of crucible + sample after drying
Five grams of the powdered samples was weighed into pre-weighed crucibles (W1) and placed into a muffled
furnace at 550°C for 8 hrs. The ash was cooled in a desiccator and weighed (W2). The weight of the ash
was determined by the difference between the powdered sample, pre-weighed crucible and the ash in the
crucible. Percentage ash was calculated as:
Where:
W1 = Weight (g) of empty crucible
W2 = Weight (g) of crucible + ash
One gram of the powdered sample was placed in a beaker and boiled in 150cm3 of 1.25% H2SO4 solution
for 30 min. The boiled sample was washed 3 times with 30cm3 of hot deionised water and filtered through
Whatman No. 1 filter paper. The residue was scrapped back into the beaker with a spatula and boiled again
in 150cm3 of 1.25% NaOH solution for another 30 min. The boiled sample was washed as in the acid
digestion but the last wash was done with cold deionised water, and washed three times with 25cm3 of
acetone and filtered as above. The residue was carefully transferred into a weighed crucible where it was dried
in the oven at 105°C to a constant weight (W1). It was thereafter burnt to ash in a muffle furnace at 550°C,
cooled in a desiccator and weighed (W2). The percentage crude fibre was calculated as:
Where:
W1 = Weight (g) of crucible + sample after washing and drying
W2 = Weight (g) of crucible + sample ash
Crude fat was determined by solvent extraction gravimetric method described by Ilodibia et al. (2014) [19].
Two gram of the powdered sample was wrapped in a Whatman No. 1 filter paper and put in a thimble. The
thimble was put in a soxhlet extractor and extracted into a pre-weighed extraction flask containing 200cm3
of petroleum ether.
The upper of the reflux flask was connected to a water condenser. The solvent (petroleum ether) was heated, boiled, vaporised and condensed into the reflux flask filled. Soon the sample in the thimble was covered with the solvent until the reflux flask filled up and siphoned over, carrying its oil extract down to the boiling flask. This process was allowed to go on repeatedly for 4 hrs before the defatted sample was removed, the solvent recovered and the oil extract was left in the flask. The flask (containing the oil extract) was dried in the oven at 60°C for 30 min to remove any residual solvent. It was cooled in the desiccator and weighed. The weight of fat extract was determined by difference and calculated as a percentage of the weight of sample analyzed thus:
Where:
W1 = Weight (g) of empty extraction flask
W2 = Weight (g) of flask + oil (fat) extract
Crude protein was determined using the micro-Kjeldahl whereby 2g of sample was weighed along with
20cm3 of distilled water into a micro-Kjeldahl digestion flask [20]. It was shaken and allowed to stand for
some time. Fifteen grams of NaSO4 and 1g of CuSO4 as catalysts were added followed by addition of 20cm3 conc. H2SO4. Some glass beads were added as anti-bump. The flask was heated under a fume cup board
for 4 hrs and then allowed to cool. The content was transferred into a 50cm3 volumetric flask and diluted to
the mark with water. An aliquot of 10cm3 of the digest was transferred into another micro-Kjeldahl flask
along with 20cm3 distilled water and placed in the distilling outlet of the micro-Kjeldahl distillation unit.
A conical flask containing 20cm3 of boric acid indicator was placed under the condenser outlet. A 20cm3
of 40% NaOH solution was added to the content in the Kjeldahl flask by opening the funnel stop cock.
The distillation started and the heat supplied was regulated to avoid sucking back. When all the available
distillate was collected in 20cm3 boric acid, the distillation stopped.
The nitrogen in the distillate was determined by titrating with 0.01M of H2SO4. The nitrogen content of the sample is given by the formula:
Where:
TV = Titre value of acid (cm3);
Na = Concentration or normality of acid;
V1 = Volume of distilled used for distilling the digest (50cm3);
V2 = Volume of aliquot used for distilling the digest (10cm3); and
G = Weight of sample (2g).
The crude protein was calculated as % Crude Protein = % N x 6.25
The carbohydrate content was obtained by subtracting the values of moisture, ash, crude fibre, crude fat and
crude protein from 100. Thus;
Acid digestion of the samples was carried out following Antia et al. (2006) [21]. Aqua reader was prepared
by adding 133cm3 of nitric acid to 400cm3 of hydrochloric acid in a 2L measuring cylinder. Distilled water
was then added to the acid to the mark of 2L to make the aqua reader. In a beaker, 30cm3 of the aqua reader
was added to 0.5g of the sample and boiled on hot plate and the sampled was completely digested. The
digest was allowed to cool and then filtered into a 50cm3 volumetric flask through Whatman No. 1 filter
paper. Distilled water was added to the filtrate to make it 50cm3 and stored in sampling bottles. The digested
samples were used for determination of sodium (Na), potassium (K), calcium (Ca), magnesium (Mg) and
phosphorus (P).
All data were collected in triplicates and subjected to analysis of variance (ANOVA) at 5% level of significance
using Graph Pad Prism (version 7.03). Significantly different means were separated by using Bonferroni’s
multiple comparisons test and Fisher’s LSD. All analyses were carried out at p < 0.05.
Results
The moisture level in grains treated with E. balsamifera varied between 10.15 ± 0.01 and 10.24 ± 0.01%. In
L. inermis and M. hirtus treatments, it ranged from 10.19 ± 0.00 to 10.20 ± 0.01% and 10.20 ± 0.01 to 10.22
± 0.01%, respectively. Grains treated with S. obtusifolia had the highest moisture level which varied between
10.21 ± 0.01 to 10.25 ± 0.01%. This was closely followed by permethrin with 10.24 ± 0.02% moisture.
However, the untreated grains had 10.27 ± 0.07% moisture (Table 1). The difference in moisture content
was highly significantly different (F (6, 42) = 5260.00, p < 0.0001), while it was not significantly different
among concentrations (F (2, 42) = 0.8098, p = 0.4518).
Ash content of grains treated with the botanical powders varied with difference in botanical type at the concentrations, 2.5, 5.0 and 10.0 x 104ppm. The ash content was in decreasing order of Permethrin > E. balsamifera > L. inermis > M. hirtus > S. obtusifolia > control. A significant difference existed among the botanical powders as well as among the varying concentrations.
Untreated and infested grains (control) had the highest percent crude fibre as 13.67 ± 0.33 followed by 2.33 ± 0.33 to 4.00 ± 0.00% in S. obtusifolia. Grains treated with M. hirtus, had 2.00 ± 0.00 to 3.67 ± 0.33%, while the crude fibre in L. inermis and E. balsamifera treatments ranged from 2.00 ± 0.00 to 3.33 ± 0.33% (Table 1). From the two-way ANOVA, the difference in crude fibre was highly significant among grains treated with the botanical powders (F (6, 42) = 932.80, p < 0.0001) as well as among the varying concentrations (F (2, 42) = 24.54, p < 0.0001).
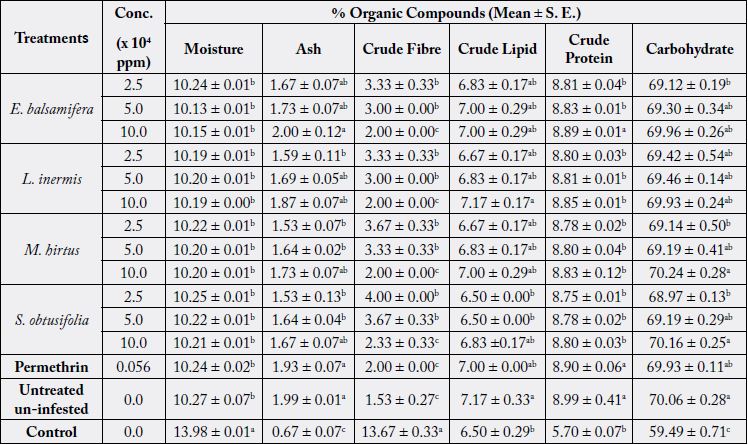
Conc. = Concentration.
Means in the same column followed by different letter superscript are significantly different at p < 0.05 by the Bonferroni’s Multiple Comparisons Test and Fisher’s LSD.
Percent crude lipid in sorghum grains treated with the botanical powders ranged as follows: 6.83 ± 0.17 to 7.00 ± 0.29% in E. balsamifera, 7.00 ± 0.29 to 7.17 ± 0.17% in L. inermis, 6.67 ± 0.17 to 7.00 ± 0.29% in M. hirtus, 6.50 ± 0.00 to 6.83 ± 0.17% in S. obtusifolia and 7.00 ± 0.00% in permethrin. The untreated uninfested and control had 7.17 ± 0.33 and 6.50 ± 0.29%, respectively. There was a significant difference (F (6, 42) = 3.198, p = 0.0112) in lipid content among the treatments while it was not significant (F (2, 42) = 1.324, p = 0.2769) in the percent crude lipid among the concentrations.
Protein content in grains treated with botanical powders at the varying concentrations differed and recorded in the following order: Permethrin > E. balsamifera > L. inermis > M. hirtus > S. obtusifolia > control. The percent protein was significantly different among the treatments. Moreover, there was no significant difference in crude protein among the varying concentrations.
Highest (70.24 ± 0.28%) percent carbohydrate was recorded in 10.0 x 104ppm of M. hirtus, while the least (69.12 ± 0.19%) was in 2.5 x 104ppm of E. balsamifera (Table 1). The untreated un-infested and control had 70.06 ± 0.28 and 59.49 ± 0.71% carbohydrate, respectively. A highly significant difference existed in the percent carbohydrate among the treatments (F (6, 42) = 310.20, p < 0.0001). Also, there was a significant difference among the varying concentrations.
There was decrease in loss of mineral elements in botanically treated sorghum grains infested by S. zeamais.
There was highly significant difference in Na (F (6, 42) = 278.80, p < 0.0001) among treatments and between
treatments and the control. Highest (26.32 ± 0.04mg) amount of Na was in 10.0 x 104ppm E. balsamifera,
while 2.5 x 104ppm of S. obtusifolia had the lowest (22.52 ± 0.04mg). Similar trend was recorded in all the
other elements (K, Ca, Mg and P) (Table 2). The amounts of Na (26.06 ± 0.18mg), K (64.45 ± 0.20mg) and
P (2.80 ± 0.02mg) in permethrin treatments were significantly the same to the highest concentration of E.
balsamifera and L. inermis. Phosphorus (P) was the least with its highest (3.12 ± 0.01mg) and lowest (2.22 ± 0.02mg) contents in untreated uninfested grains and 2.5 x 104ppm of S. obtusifolia, respectively. Loss of
the Na, K, Ca, and Mg in the control was greater than in the botanical treatments (Table 2).
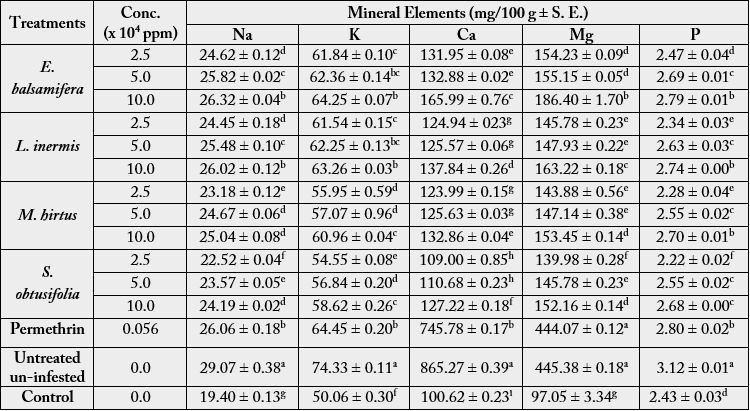
Conc. = Concentration.
Means in the same column followed by different letter superscript are significantly different at p < 0.05 by the Bonferroni’s Multiple Comparisons Test.
Discussion
There is paucity of information on the effects of botanicals on nutritional composition of sorghum grains.
However, the effectiveness of the selected botanical powders in reducing organic compounds in treated
sorghum corroborates Danjumma et al. (2009) who reported that powders of A. indica, A. sativum, N.
tabacum, O. basilicum, Z. officinale applied at 1 and 2g/50g (w/w) decreased loss of organic compounds in
treated maize grains. Effect of lower concentration of E. balsamifera and L. inermis on moisture content of
stored sorghum grains was significantly the same as permethrin powder which concurs with Danjumma et al. (2009) who reported that moisture content of maize grain treated with 2g/50g A. indica and a chemical
insecticide (coopex) was significantly the same.
The high moisture content in the control (damaged rains) corresponds to the findings of Okoroafor and Job (2017) who reported an increase in moisture content of yellow and white local maize varieties after 12 months of storage [22]. This increase in moisture content of damaged grains could be due to the fact that grain is a living organism that respires and emits moisture that moves within the grain mass. Prolonged stay of insects in the control could also increase respiratory activities in the grain and add more moisture content. This phenomenon was explained by USAID (2011) [23] that biological factors such as insects and moulds respire and can add to the moisture being released and migrating through the stack.
Ash content of the infested grains was significantly affected when treated with different botanical powders. Grains treated with permethrin lost little amount of ash, while in the control it was high. Similar results were reported by Danjumma et al. (2009) when maize grains were treated with coopex. The loss of ash content in sorghum treated with the selected botanicals was concentration dependent. This shows that the weevil’s feeding and reproductive activities might have reduced the mineral content of the grains [14].
It was found that the untreated sorghum grains had more crude fibre than the treated grains, which concurs with Danjumma et al. (2009) who reported that maize grain treated with botanical powders of A. indica, A. sativum, N. tabacum, O. basilicum and Z. officinale had less crude fibre than the untreated grains. The increase in fibre content in untreated infested grains was as a result of feeding activities of the weevils on endosperm hollowing out the grain leaving only the bran, which is largely fibre. This finding agrees with Bamaiyi et al. (2006) who noted that insect infestations decreased the nutritional quality of grains and increased the relative level of dietary fibre [14]. Botanical concentration also affected crude fibre level in the stored sorghum. Higher concentration seemed to preserve more nutritional quality as there were low infestations, hence lower level of fibre than grains treated with lower concentration.
Loss in lipid content in sorghum grains was significantly reduced by the selected powders at the various concentrations. This is consistent to Danjumma et al. (2009) who found more crude fat in maize grains treated with some botanical powders than in untreated control [13]. There was drastic loss in crude lipid in untreated sorghum compared to the treated grains, supporting Jood et al. (1996) [24] that insect infestations resulted in substantial reduction in crude lipid content of stored grains. There was a reduction in crude lipid in white and yellow local maize varieties damaged by S. zeamais after 12 months of storage [22]. The reduction in crude lipid of untreated grains might probably be due to mass infestations by S. zeamais which were actively feeding on germ and endosperm of the grain. It was observed that loss in crude lipid increased with increased levels of insect infestations [14].
The decrease in total protein content in sorghum grains treated with botanicals of E. balsamifera, L. inermis, M. hirtus and S. obtusifolia was less than in untreated grains. It was found that higher concentration of E. balsamifera and L. inermis were the most effective. This was because the botanicals must have killed most of the weevils early before feeding on the grains, and served as antifeedants to the few that survived shortly, while in the control they survived for longer period and fed much on the grains. Therefore, as insect infestations increased, feeding activity increased and probably a decrease in the protein content of grains. Bamaiyi et al. (2006) [14] found that the endosperm component of maize grains contains 90% of seed protein, which is readily damaged by insects, and sorghum has protein content ranging from 7 to 15% [9]. Crude protein in white local maize was reduced from 9.65% to 8.86% in undamaged and damaged grains, respectively [22].
Carbohydrate content in sorghum grains was affected by the weevil’s infestations and it was found that the selected botanicals reduced its loss. The untreated grains lost more carbohydrate than the treated ones, probably due to the fact that the insects were freely feeding on the untreated grains while the botanicals inhibited feeding and hence reduced feeding activities. Carbohydrates are important components of the diet for most insects as usual respiratory fuels, converted to lipid, provide carbon skeleton for the synthesis of various amino acids and the cuticle characteristically contains the polysaccharide chitin [25]. Consumption of more carbohydrate in the untreated grains was observed by Chapman [26] that insects feeding on stored products can use a wide range of carbohydrates.
There was substantial decrease in mineral content in infested grains compared to the botanical treatments.
This could be due insect feeding which was hindered in the botanical treatments as a result of their
antifeedant as well as toxicity effects against the weevils. This is in agreement with Jood and Kapoor (1993)
[27] and Danjumma et al. (2009) who found that feeding by S. zeamais reduced the minerals content of
maize grains. The minerals content also depended on botanical concentrations that higher amounts of the
powders preserved more minerals than lower concentrations. It was found that permethrin powder was the
most effective in reducing loss of mineral elements in infested sorghum grains followed by E. balsamifera, L.
inermis, M. hirtus and S. obtusifolia. Similar results were reported by Danjumma et al. (2009) that botanical
powders of A. indica, A. sativum, N. tabacum, O. basilicum and Z. officinale at different concentrations preserved
more Na, K, Ca, Mg and P in infested maize grains than in untreated infested grains.
Conclusion
Findings of this study have revealed that leaf powders of E. balsamifera, L. inermis, M. hirtus and S. obtusifolia
reduced losses in proximate composition and mineral contents of sorghum grains and hence, could serve
as alternative means protecting sorghum from qualitative damage due to S. zeamais infestations which may
probably contribute in solving cases of malnutrition especially in rural areas.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!