Biography
Interests
Dr. Okudu, H. O.* & Dr. Asumugha, V. U.
Department of Human Nutrition and Dietetics Michael Okpara University of Agriculture Umudike, Abia State
*Correspondence to: Dr. Okudu, H. O., Department of Human Nutrition and Dietetics Michael Okpara University of Agriculture Umudike, Abia State.
Copyright © 2018 Dr. Okudu, H. O., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The yellow (C. lepidota) and white (C. parchycarpa) monkey kola varieties were collected from the major markets in Calabar (South-south) and Umuahia (South - east) Nigeria. The pulps (edible part) were carefully cut longitudinally and then separated from the seed. The seeds were grated with a kitchen grater. Samples were dried for three days using locally constructed solar dryer. Milling the dried seeds into flour was done using attrition milling machine. The proximate compositions of the sample were determined using AOAC methods. Mineral elements were determined using wet-acid digestion method for multiple nutrients determination as described by the method of AOAC. The ß - carotene, riboflavin, niacin and thiamin values of the products were determined using spectrophotometry method, while ascorbic acid was determined using titration method. All tests were carried out in duplicates and the data generated were analysed using standard methods. Crude protein (6.3 - 9.0g/100g), crude fat (4.4 - 4.8g/100g), Ca (228 - 373mg/100g), K (105.6 - 121.5mg/100g) and Mn (238 - 349mg/100g) while ash (4.2 - 6.6g/100g), Mg (39.5 - 54.7mg/100g), Fe (29.1 - 38.4mg/100g) and ß – carotene (15,729 - 39,405mcg/100g) of C. parchycarpa seed were significantly (p<0.05) higher than that of C. lepidota seed. Both seeds had substantial amounts of phytochemicals (particularly flavonoid, alkaloid and saponin). The high phytochemical values obtained in monkey kola seed makes it an important seed that need to be fully exploited.
Introduction
Fruits and vegetables are an important component of a healthy diet. Their inclusion in the daily diet not
only serve as cheap source(s) of vitamins and minerals but a means of reducing risk for some forms of
cancer, heart disease, stroke, and other chronic diseases [1-3]. In Nigeria a variety of fruits and vegetables are
consumed on a daily basis, but most often only the fleshy pulp of these fruits are consumed leaving the seed
and the rind. Studies carried out on some seeds showed that they are rich sources of vitamins, fibres, minerals
and other essential nutrients activity than the pulp fractions [4,5]. The present uncertainty in the world food
supply and the expected increase in demand have warranted the search for alternative sources of food which
will be readily available for all and sundry by the government planners and scientists.
Monkey kola is grouped among the wild fruits in Nigeria. It is a member of the family of sterculiaceae and belongs to a group called drupes [6]. It is made up of three varieties: red (Cola latertia), yellow (Cola parchycarpa) and white (Cola lepidota) [7]. The pod of the yellow variety is roundish, while the white variety has more cylindrical shape. Monkey kola is identified by various local names in Southern Nigeria (“achicha” or “ohiricha” in Igbo and “ndiyah” in Efik). Monkey kola is cultivated throughout the tropical regions of the world; it is commonly found in Southern Nigeria between the months of June to November [8].
Contributions of monkey kola to intake particularly among the rural populace and the urban poor have been documented [6,7]; like most fruits only the pulp of monkey kola is utilized as food while the seeds are discarded as waste. Knowing the chemical composition of monkey kola seed may warrant its utility as an alternative source of food or animal feed. This work was therefore designed to evaluate the chemical composition of two varieties of monkey kola seeds (Cola parchycarpa, Cola lepidota).
Materials and Methods
The two varieties monkey kola; yellow (Cola parchycarpa) and white (Cola lepidota) were identified botanically
in the Department of Forestry, Michael Okpara University of Agriculture, Umudike, Abia State. Nigeria. The
yellow and white varieties were collected from the major markets in Calabar (South-south) and Umuahia
(South - east) zones of Nigeria. Samples were purchased from at least five randomly selected vendors in the
various markets and pooled to obtain the samples for the study.


The fruits were inspected and sorted. Fruits that were firm, matured and free from insect damage or
mechanical injuries were selected. The outer covering of the fruits were cut open using a kitchen knife and
stripped/peeled off manually. The pulps (edible part) were carefully cut longitudinally and then separated
from the seed. The seeds were grated with a kitchen grater. Samples were dried for three days using locally
constructed solar dryer. Milling the dried seeds into flour was done using attrition milling machine (Thomas
Wiley Model ED-5) to pass through 5mm sieve size. The milled samples were package in air-tight polythene
for chemical analysis.
The proximate compositions of the sample were determined using standard AOAC (2006) methods. Moisture
content of the seed was determined gravimetrically [9]. The crude protein content was determined by micro-
Kjeldahl method, using 6.25 as the nitrogen conversion factor. The crude fat content was determined by
Soxhlet extraction method using petroleum ether. The ash content was determined by incinerating the
samples at 600°C in a muffle furnace. Carbohydrate was obtained by difference, while energy was calculated
using the Atwater Conversion factors in KJ and Kcal (17KJ/4Kcal, 17KJ/4Kcal, and 37KJ/9Kcal, for protein,
carbohydrate and lipid respectively.
Mineral elements were determined using wet-acid digestion method for multiple nutrients determination as described by the method of AOAC (2006) [9]. About 0.2g of the processed sample material was weighed into a 150ml Pyrex conical flask. Five (5.0) ml of the extracting mixture (H2SO4 - Sodium Salicylic acid) was added to the sample. The mixture was allowed to stand for 16 hours. The mixture was then placed on a hot plate set at 30°C and allowed to heat for about 2hours. Five (5.0) ml of concentrated perchloric acid was introduced to the sample and heated vigorously until the sample was digested to a clear solution. Twenty (20) milliliters of distilled H2O was added and heated to mix thoroughly for about a minute. The digest was allowed to cool and was transferred into a 50ml volumetric flask and made up to the mark with distilled water. The digest was used for the determinations of calcium (Ca) and magnesium (Mg) by the ethylamine ditetra acetic acid (EDTA) versanate complexiometric titration method.
Potassium (K) and sodium (Na) were evaluated by flame photometry method and phosphorus (P) by the vanadomolybdate method using the spectrophotometer. The trace metals (zinc, iron, copper, selenium, manganese and iodine) were determined using the atomic absorption spectrophotometer 969 instrument. The appropriate cathode lamp was fixed for each element. The sample was introduced to the atomizer and the value concentration of the element printed out as mgX/liter.
The ß-carotene, riboflavin, niacin and thiamin of the products were determined spectrophotometrically as described by AOAC (2006) [9]. Ascorbic acid was determined as described by AOAC (2006) using titration method [9]. Gravimetric method was used to determine alkaloids [10]. Saponin was determined by gravimetric oven drying method as described by the method of AOAC (2006) [9]. Tannin content of the sample was determined using the method of spectrophometry as described by Kirk and Sawyer (1991) [11]. Phenol was determined by the folin-ciocatean spectrophotometry method (AOAC 2006) [9]. Flavonoid was determined by gravimetric oven drying method as described by Harborne (1973) [10].
All determinations were done in duplicates. The data generated were entered into the computer and analyzed
using IBMSPSS (version 20) Means and standard deviations were calculated from the chemical analysis
data. Analysis of Variance (ANOVA) was use to separate the means and thelevel of significance was accepted
at p<0.05.
Results
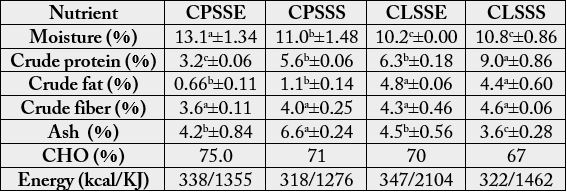
The energy and proximate composition of monkey kola seed flours are shown on Table 1. The residual
moisture obtained for Cola parchycarpa seed (11.0-13.1%) was significantly (p<0.05) higher than the residual
moisture of Cola lepidota seed (10.2-10.8%). Crude protein value varied in all the samples. C.lepidota seed
from Calabar had higher crude protein value (9.0%) compared to the other samples (3.2-5.3%).
Values are means±standard deviation of duplicate determinations.
CPSES- C. parchycarpa seed South-east, CPSSS- C. parchycarpa seed South-south
CLSSE- C. lepidota seed South-east, CLSSS-C. lepidota seed South-south
Crude lipid obtained for C. lepidota seed (4.4 - 4.8%) was significantly (p<0.05) higher than the crude lipid found in C. parchycarpa seed (0.66-1.1%) irrespective of location. It was observed that C. parchycarpa seed from Calabar had a significantly (p<0.05) higher ash content (6.62%) than the other samples (3.60-4.50%). Energy (KJ) calculated for C. lepidota seed (1462-2104KJ) was higher than the energy calculated for C. parchycarpa seed (1276-1355KJ) irrespective of location.
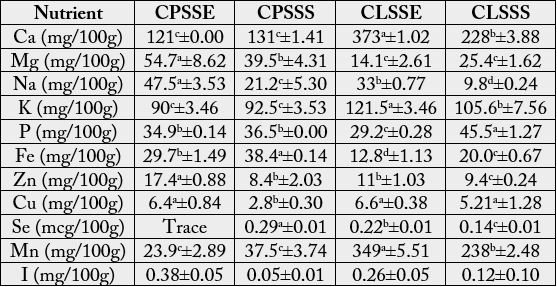
The macromineral composition of monkey kola seed on Table 2 revealed that C. lepidota seed had significantly (p<0.05) higher calcium (228- 373mg/100g) value than C. parchycarpa seed (121-131mg/100g) irrespective of location. C. pachycarpa seed from Umuahia had significantly higher magnesium and sodium contents (54.7mg/100g, 47.5mg/100g respectively) than the other samples (14.1-39.5mg/100g, 9.8- 33.05mg/100g respectively). C. lepidota seed had significantly (p<0.05) higher (105.6-121mg/100g) potassium value than C. parchycarpa (90-92mg/100g) irrespective of location. C. lepidota seed from Calabar had higher phosphorus (45.5mg/100g) than the other samples (29.2-36-5mg/100g).
Values are means±standard deviation of duplicate determinations.
CPSES- C. parchycarpa seed South-east
CPSSS- C. parchycarpa seed South-south
CLSSE- C. lepidota seed South-east
CLSSS- C. lepidota seed South-south
The micromineral content on Table 2 shows that C. parchycarpa seed had higher iron values (29.7-38.45mg/100g) than C. lepidota seed (12.8-20.0mg/100g) irrespective of location. C. parchycarpa seed from Calabar however, had a significantly (P<0.05) higher iron content (38.45mg/100g) than the seed from Umuahia (29.7mg/100g). C. parchycarpa seed from Umuahia had significantly (p<0.05) higher zinc content (17.4mg/100g) than the other samples (8.49-11.03mg/100g). Copper obtained in all the samples was generally low. C. lepidota seed had significantly (p<0.05) higher manganese (238-349mg/100g) than C. parchycarpa seed (23.9-37.5mg/100g) irrespective of location. C. parchycarpa seed from Umuahia had significantly (p<0.05) higher iodine content (0.38mg/100g) than the other samples (0.05 - 0.26mg/100g).
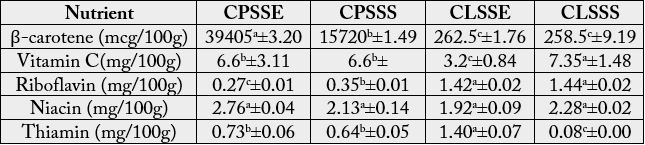
The vitamin compositions of the seeds are shown on Table 3. The ß – carotene obtained from C. parchycarpa
(1572.0 -3940.5mcg/100g) was significantly (p<0.05) higher than the ß – carotene content of C. lepidota
(258.5 - 262.5mcg/100g) irrespective of location. The vitamin C content of C. lepidota from Calabar
(7.35mg/100g) was significantly (p<0.05) higher than those of the other seeds (3.20 - 6.60mg/100g).
Values are means±standard deviation of duplicate determinations.
CPSES- C. parchycarpa seed South-east
CPSSS- C. parchycarpa seed South-south
CLSSE- C. lepidota seed South-east
CLSSS- C. lepidota seed South-south
The riboflavin obtained for C. lepidota (1.42 -1.44mg/100g) was significantly (p<0.05) higher than that of C. parchycarpa (0.27- 0.35mg/100g) irrespective of location. There was however no significant (p>0.05) difference in the niacin content of C. lepidota seed (1.92 - 2.28mg/100g) and that of C. parchycarpa seed (2.13- 2.76mg/100g). The thiamin obtained for C. lepidota from Umuahia (1.40mg/100g) was significantly (p<0.05) higher than the thiamin content of all the other samples (0.08- 0.73mg/100g).
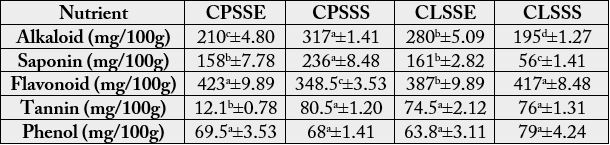
The phytochemical composition of the two varieties of monkey kola (C. parchycarpa, C. lepidota) seeds
is shown on Table 4. The alkaloid and saponin content of C. parchycarpa seed (210 - 317mg/100g and
158 - 208mg/100g respectively) were significantly (p<0.05) higher than those of C. lepidota seed (195 -
208mg/100g, 56 – 161mg/100g). The flavonoid content of C. parchycarpa was 348.5 - 423mg/100g while
that of C. lepidota was 387 - 417mg/100g. The flavonoid content of C. parchycarpa seed from Umuahia
(423mg/100g) was similar to that of C. lepidota seed from Calabar (417mg/100g).
Values are means ±standard deviation of duplicate determinations.
CPSES- C. parchycarpa seed South-east
CPSSS- C. parchycarpa seed South-south
CLSSE- C. lepidota seed South-east
CLSSS- C. lepidota seed South-south
The tannin content of C. lepidota (74.5 - 76mg/100g) was similar to that of C. parchycarpa seed from Calabar (68mg/100g) but significantly (p<0.05) higher than the tannin content of C. parchycarpa seed from Umuahia (12.1mg/100g). There was no significant difference between the phenol value of C. parchycarpa seed (68 - 69.5mg/100g) and that of C. lepidota seed (63.8 - 79mg/100g).
Discussion
The study showed that monkey kola seed is a good source of plant protein (particularly C. lepidota seed),
and crude fiber but a poor source of crude lipid. There is record of work done on monkey kola seed [11],
but unfortunately the values obtained in that study cannot be compared with this present study because
of the differences in the mode of expression of unit. However, when the values obtained in this study were
compared with other works, the crude protein content of monkey kola was lower than that of mango seed
(10.06%) but the crude fiber and ash obtained in this study were higher than the crude fiber (2.40%) and
ash (2.62%) reported for mango seed [5]. Fiber in diet is associated with health benefits; it aids in weight
control and reduces the risk of developing obesity [12]. The crude fat in monkey kola seed (particularly in C.
parchycarpa) was low. The low fat value obtained in this study was not surprising because seeds are generally
poor sources of fat with the exception of oil seeds or groundnuts [13]. Most seeds contain between 1-5%
fat; this implies that monkey kola seed fat value conformed to the general seed values. The carbohydrate
obtained for the two varieties of monkey kola were not significantly different from each other, but the energy
value of C. lepidota seed was significantly (p<0.05) higher than that of C. parchycarpa seed irrespective of
location. The higher energy obtained for C. lepidota seed is a function of its composition [14].
The most abundant macromineral found in monkey kola seeds were calcium and potassium. The values of calcium and potassium were many folds higher that the values reported for most seeds [15]. These two macronutrients are known for their vital roles in the body [14]; potassium is needed in the body for normal nerve and muscle function and in the maintenance of acid-base of the body [16,17] and calcium is an important constituent of bones and teeth. Other minerals found in considerable quantity include: Mg, Na, Fe, Zn, Se and I for C. pachycarpa seed and P, Cu, Mn for C. lepidota seed.
Monkey kola (particularly C. parchycarpa variety) is a good source of ß-carotene. The ß-carotene content of C. parchycarpa seed was higher than the ß-carotene content of pawpaw (966mcg/100g), mango (708 - 4720mcg/100g) and that of sesame seed (50mcg/100g). Apart from being a pro-vitamin ß-carotene is also linked with antioxidant activities [18]. Monkey kola seed also had substantial quantity of vitamin C and the B-vitamins (thiamin, riboflavin, niacin). The vitamin C content of monkey kola seed was many folds higher than that of most seeds while the B-vitamins were comparable to those most common seeds [15]. Vitamin C plays significant role in the synthesis of several important compounds while the B-vitamins are known are for their role in energy metabolism [19].
Flavonoids, alkaloid and saponin were the major phytochemical found in the seed. Other phytochemicals found in the seed were tannin and phenol. The phytochemical values obtained for monkey kola seed were higher than the values reported for most common seeds [15]. Among the important classes of phytochemicals are the flavonoids and phenol (Cassidy and Dalais, 2003). There is in recent years an increasing evidence also of the health benefits of alkaloid, saponin and tannin [20-23].
Conclusion
The study showed that C. lepidota seed had significantly higher crude protein, crude fat, calcium, potassium
and manganese while C. parchycarpa seed had significantly higher ash, magnesium, iron and ß-carotene.
Both seeds had substantial amounts of phytochemicals (particularly flavonoid, alkaloid and saponin). The
high phytochemical values obtained in the two varieties of monkey kola seeds calls for the need of the seeds
to be fully exploited.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!