Biography
Interests
Rojamadhuvanthi, C.1 & Dr. Anne Rebecca, A.2*
1Department of Zoology, PSG college of Arts and Science, Coimbatore, Tamil Nadu, India
2Assistant Professor, Department of Zoology, PSG college of Arts and Science, Coimbatore, Tamil Nadu, India
*Correspondence to: Dr. Anne Rebecca, A., Department of Zoology, PSG college of Arts and Science, Coimbatore, Tamil Nadu, India.
Copyright © 2018 Dr. Anne Rebecca, A., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The gut microbial community is one of the richest and most complex ecosystems on earth. Aquaculture
industry of the world is facing serious problems due to microbial diseases of pathogenic microbes.
On the other hand, probiotics are healthy gut microbiota and play an important role in host
development and immune system protection. Macrobrachium malcolmsonii is a second largest fast
growing prawn occurs commonly in Indian rivers, draining into the Bay of Bengal. M. malcolmsonii
is also abundant in the river Cauvery, a major perennial river of southern India. Phytoplaktons and
zooplanktons are biological indicators whereas, water quality parameters determine the diversity
of gut microbiota.
Qualitative and Quantitative analysis of gut microbiota were carried out in adult M. malcolmsonii
of length (25.09±3.25)cm and weight (12.05±0.52)gm, within 36 hours of capture from the wild.
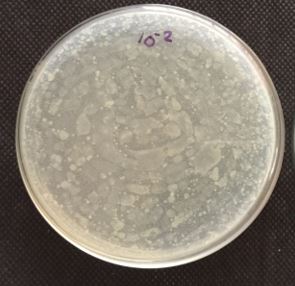
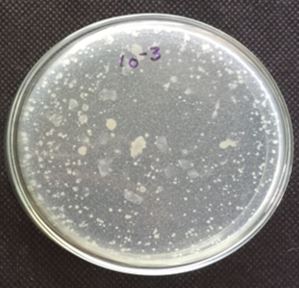
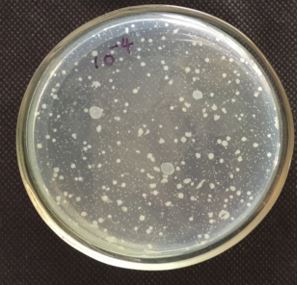
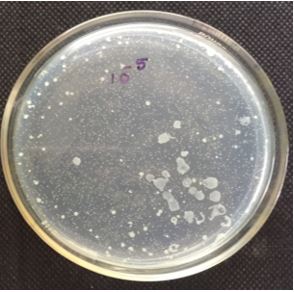
The quantification of 10-5 dilution resulted in 86 colonies. Qualitative examination of gut microbiotia
reaveled the presence of bacteria belonging to the genera Escherichia, Bacillus, Pseudomonas,
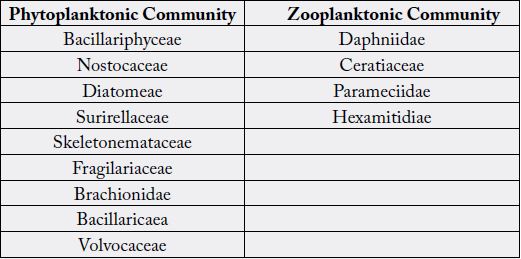
Salmonella, Vibrio and Staphylococcus. Bacillariphyceae, Nostoceae, Diatomea, Surirellaceae, Skeletonemataceae,
Fragilariaceae, Brachionidae, Bacillaricaea and Volvocaceae families of phytoplanktons
and Daphniidae, Ceratiaceae, Parameciidae and Hexamitida families of zooplanktons where
the predominating planktonic community in water medium of the host species. Water temperature
of 26±1°C, PH 7.3±0.2, dissolved oxygen 3.72±0.23, alkalinity 15.33±7.69, salinity 0.053±0.001
and chlorides 16.2±1.73 were recorded for the water medium of the host species.
Significant increase in alkalinity is attributed to the gut associated microbiota, with distribution of
pathogenic groups.
Background
Crustacean aquaculture is a very important commercial activity in several countries of Asia and the Americas.
Aquatic ecosystem is known to support the range of organisms. A bacterial species of the gut can influence
the health of the host. Freshwater prawn (Macrobrachium malcolmsonii), the second largest fast growing
prawn occurs commonly in Indian rivers, draining into the Bay of Bengal. M. malcolmsonii [1] and is an
omnivorous bottom dwelling freshwater prawn. Several microbial pathogens are known to cause disease in
cultured crustaceans and these have been reviewed by several workers [2]. Feeding habits, immune system,
ontogenic characters are the some of the factors affecting the gut microbiota [3]. Gut microbiota is found to
cluster with environmental microbiota and environmental influences [4]. Planktonic population observation
may be used as a reliable tool for bio-monitoring studies to assess the pollution status of aquatic bodies [5].
Freshwater zooplanktons play an important role in ponds, lakes and reservoirs ecosystem and food chain [6].
Presence of E. coli and pathogenic gut microbiota as Salmonella Sps. is ascertained to fecal contamination
and environmental pollution [7]. An investigation on gut microbiota of M. malcolmsonii was performed and
to characterize the presence of pathogenic and non-pathogenic microbiotic communities and determine
their causative factors by assessing the biological indicators and abiotic components of the medium.
Materials and Methods
Adults of M. malcolmsonii (12.05±0.52cm in length and 25.09±3.25gm in weight) were used for the study.
The samples were collected from the lower anicut of Cauvery, Anakkari, packed with ice to maintain minimal
temperature ambience and transported to the laboratory, examined for gut microbiota within 36 hrs.
Intestine of the specimen was removed by using sterile scalpel, forceps and sterile knife. 5gm of each
dissected gut tissue was homogenized with 100ml of 0.85% of NaCl. The resulting homogenate was serially
diluted at 10-2, 10-3, 10-4 and 10-5 respectively with 0.85% saline. From the diluted sample around 1.0ml
of the sample were transferred to petriplate along with 20ml of the nutrient agar was and is rotated both
clockwise and anticlockwise. After solidification the plates were incubated at 37°C for 24 hrs and the total
number of colonies were calculated. The total bacterial densities were enumerated by spread plate method
by using sterile instruments.
The isolates were inoculated over nutrient agar, skim milk agar, Simmons citrate agar, Mannitol salt agar,
Mac Conkey agar, triple sugar iron agar, cetrimide agar and TCBS incubated at 37°C for 24 hours.
The water samples were collected from the lower Anicut reservoir. Sampling was done at two different
stations of the lower Anicut. Temperature and pH of the water samples were recorded at the site. Random
samples of water medium were collected for the study of phytoplanktons, zooplanktons, dissolved oxygen,
salinity and alkalinity. Samples for dissolved oxygen were fixed at the site and the water samples were
transported in sterile bottles for further analysis in laboratory.
Results
Table-1 represents the enumerated of bacterial isolates in serially diluted gut homogenate. Table-2 represents
the qualitative characterization of microbiota. The results of water quality parameters were represented in
Table-3. Table-4 represents the distribution of planktonic communities in water medium.

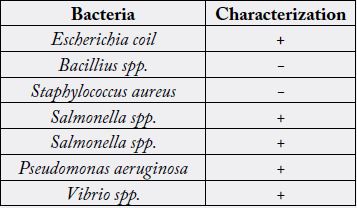
+Indicating presence of organisms; - Indicating absence of organisms
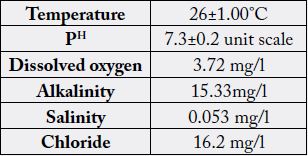
The results are mean value of triplicate analysis ± standard deviation.
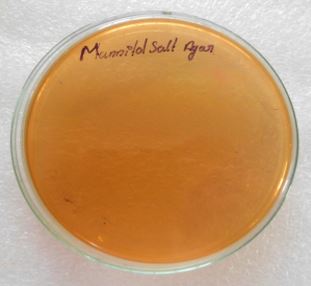
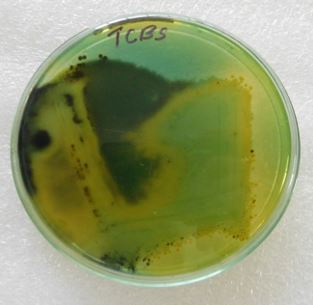
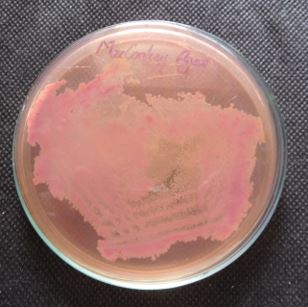
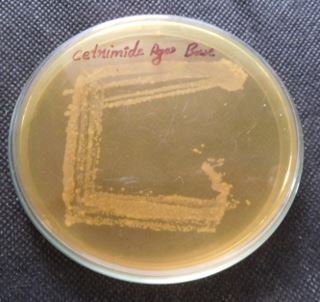
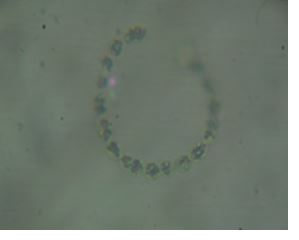


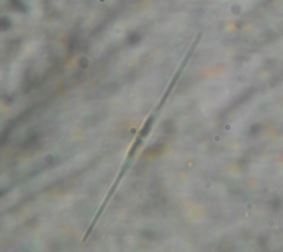
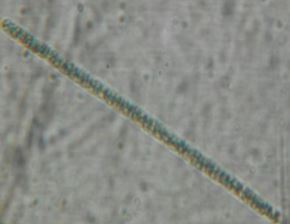
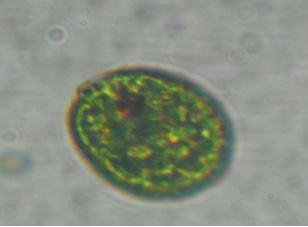
Discussion
The intestinal microbiota of animals and humans have attracted much recent attention as it is believed to
be a key factor in numerous animal functions including health, growth and disease status [9]. The influence
of the gut flora on the host is clearly of great interest in aquaculture, particularly the poor productivity [10].
Marcel et al., (2013) [11] isolated more than one species of bacteria from a single fish, and it is associated
with the nature of feed given to the fish, location of sampling sites, nearby human activities and water quality
at the fish culture area. Pakingking et al., (2015) [12] reported that the bacterial flora of the fish also reflects
the bacterial composition and health status of fish. The significant roles of fish microbiota are to protect the
host against pathogenic challenge by production of antagonistic factors, inactivation of pathogenic bacterial
toxins or metabolites, stimulation of host immunity and competition with pathogens for attachment sites
or nutrients. The pathogenic diseases are usually caused by bacterial species which are facultative pathogenic
for both fish and human. In the present study, the species M. malcolmsonii were collected and gut analysis
is carried out for the isolation and characterization of bacteria. Characterization of gut microbiota in M.
malcolmsonii, revealed the presence of bacteria belonging to the genera Escherichia, Bacillus, Pseudomonas,
Salmonella, Vibrio and Staphylococcus were identified. Similiarly, a study on bacterial flora is observed in the
digestive system of freshwater prawn M. rosenbergii showed that the E. coli is the prominent bacteria, a
reflection on the bacterial flora of the water is suggested by [13]. Vanderzant et al., (1970) [14] reported S.
aureus, Pseudomonas aeruginosa, Enterobacter, Aeromonas and Vibrio sps. as the dominant flora bacteria with E.
coli which occupies a less niche in a pond rearing system. E. coli is a Gram-negative, facultatively anaerobic,
rod-shaped, coliform bacterium of the genus Escherichia. E. coli is often non-pathogenic, although different
strains may cause diseases in gastrointestinal, urinary, or central nervous systems in fish reported by Nataro
et al., (1998) [15].
Salmonella is a gram negative, rod shaped, pathogenic bacteria of water bodies. Salmonella belongs to Enterobacteriaceae family. Salmonella enteritidis is the main culprit in fishes. The major part of the Salmonella sps. are aquatic environment species; however, fish and fishery products have been renowned as a carrier of food-borne pathogens were reported by Salmonella survival in water depends on biological (macro and micro invertebrate) and physical factors (e.g. temperature). Salmonella is associated with organic loading of the aquatic environment and poor water quality [16]. Vibrio species are part of the natural microbiota of wild and cultured prawns and become the pathogens for the natural defense mechanisms of the prawn [17]. The high prevalence of Vibrio, particularly in the gut, reinforce the opinion while members of this genus are pathogenic, they are not primary pathogens which exist in and around prawns in the environment as part of their normal microbiota [18]. However, Somework on disease syndromes of penaied shrimp have been caused by vibrio species which behave more like true pathogens than opportunistic invaders [19]. Thus, the Vibriosis is controlled by vigorous water management and sanitation to prevent the entry in the culture water and to reduce stress on the prawns [20].
Bacillius species has been isolated from the gut of marine fish and applied as probiotics. It is a non-pathogenic bacterial species. The term probiotic is currently used to name ingested microorganisms associated which is benefits for humans and animals [21]. Many Bacillius strains isolated from marine fish could inhibit potential pathogens [10]. Pseudomonas spp. population in the gut of healthy adult freshwater prawn, were observed by [22]. Pseudomonas is often encountered in sea water, sediments, phytoplankton and zooplankton [23].
Pseudomonas shows the highest production in the amylase activity in rocky crab (Plagusia dentipes) were reported by [24]. Staphylococcus aureus is known as the enterotoxin producing agent and a microorganism which is poisonous [25].
Water quality is an important aspect in aquaculture system. Non-optimum water physico chemical parameters (dissolved oxygen, pH, salinity, ammonia, temperature etc.) and poor management practices (overfeeding, inadequate nutrition, overcrowding etc.) can cause stress to the cultured fish and thus make them more susceptible to disease outbreaks [26]. The parameters like temperature, pH, dissolved oxygen, alkalinity, salinity and chlorides were estimated from the water sample.
Fishes are poikilotherms and the body temperature depends on their external environment. Ibrahim et al., (2004) investigated the effect of temperature on the in vitro adhesive ability of potential fish probiotics [27]. Ismaila et al., (2016) performed a comparative study on water quality parameters (temperature, dissolved oxygen, ph, ammonia) between Terengganu river and Pedu lake were reported a significant influence of high water temperature associated with higher number of bacterial isolates from eyes, kidney and brain of cage cultured red hybrid tilapia fish [28]. Temperature is an important physical factor, which influence the other hydrological parameters. In this study, the maximum water temperature (26±1°C) was recorded, where E. coli can grow and divide in a wide range of temperatures (20–40°C). The minimum temperature reported for the growth of Salmonella sps. is 6.2°C [29]. The climatic warming is one of the reported factor for pathogenic association in water medium [30]. In this study pH (7.3±0.2) was recorded. Escherichia coli, staphylococci, and Salmonella sps. will grow optimally at pH 7. Similarly, the value of pH 7.6 was recorded by Nambirajan et al., (2012) at lower Anicut Thanjavur District Tamilnadu [29]. Dissolved oxygen is an important chemical factor for respiration which would get influenced by aquatic organisms. The dissolved oxygen of water sample was measured. In this study, the value of dissolved oxygen (3.72±0.23) was recorded from the water sample. Vibrio and Pseudomonas species correlates with the concentration of dissolved oxygen from the water sample. However, Dissolved oxygen is not found to be a significant factor affecting the distribution of bacterial strains in cage cultured red hybrid tilapia, Oreochromis niloticus and O. mossambicus as reported by [28]. Salinity is the total concentration of dissolved ions in the freshwater. In this study, the value of salinity is (0.053±0.001) was recorded from the water sample, which is compared with normal freshwater value. Alkalinity of water is a major of its capacity to neutralize acids. In this study, the value of total alkalinity is estimated (15.33±7.69) were recorded from the water sample. Chlorides is the indicators of contamination with animal and human waste. The estimation of chloride is estimated by using Mohr’s titration method. In this study, the value of chloride is estimated (16.2±1.73) were recorded from the water sample. In this study, the presence of alkalinity is much significant for the growth of bacteria. Physico chemical parameters of the water may influence the density of bacterial population [31]. This study revealed that the physico chemical parameters which influence the presence of various bacteria from the freshwater prawn M. malcolmsonii. However, the reports of chlorides, salinity, alkalinity on gut associated microbiota in aquatic species is limited. Similarly, all measured water quality parameters showed their importance in influencing the occurrence of bacteria. Water quality have been identified and discussed as important factors in influencing the presence of non-pathogenic and pathogenic bacteria.
Major sources of water-borne pathogens includes: domestic animals, wildlife, and humans [32]. Escherichia coli, a Thermophilic coliform found in all mammal faeces. E. coli survives in drinking water for between 4 and 12 weeks, depending on environmental conditions (temperature, microbiota, etc.). Under the conditions in distribution systems, E. coli will be much more long-lived [33]. Pathogenic gut microbiota as Salmonella Sps. is ascertained to fecal contamination and environmental pollution [7]. The principal habitat of Salmonella is the intestinal tract of humans and animals [33]. Salmonella are constantly found in environmental samples, because they are excreted by humans, pets, farm animals, and wild life. Municipal sewage, agriculture pollution, and storm water runoff are the main sources of these pathogens in natural waters [1]. Salmonellae do not seem to multiply significantly in the natural environment, but they can survive several weeks in water and in soil if conditions of temperature, humidity, and pH are favorable [33]. However, there is no published data on environmental pollution of the water medium due to industrial contaminants. Inspite, agricultural practices are one of the major livelihood of the people of Anakarai, defining the human source or agricultural source of contamination [29].
Despite the vastly different forms of interactions, symbiotic and pathogenic bacteria have in common that they are adapted to a particular environmental niche represented by the host organism or compartment thereof. Influence of biotic and abiotic fctors on symbiotic association has been reported for rhizobium sps. [36]. Dehler et al., (2017) investigated on association between gut microbiota and environmental microbiota stated a difference in microbiotic community of host gut and its environment. Many bacterial species found in the intestine were not detected at in the environmental water samples, suggesting a symbiotic mode of association with the host, which is not possible in free living state on water medium [4,37]. Phytoplankton and zooplankton is called as biological indicators. Barivona et al., (2017) reported that plankton’s diversity in fresh waters is very important because most of planktons can be used as environmental indicators [38]. Species that respond predictably to environmental conditions were used as bioindicators for particular variables of aquatic ecosystems, the dynamics of which are related to environmental changes [39,40].
Conclusion
Gut microbiota analysis of M. malcolmsonii revealed the presence of Vibrio, Salmonella, E. coli, which finds
agricultural and fecal contamination as the major environmental sources at Kollidam. However, presence
of Bacillus species is attributed to be beneficial for M. malcolmsonii as a potent probiotic species enhancing
immune tolerance to these pathogenic groups. High alkalinity of the water medium favours survival of the
pathogenic as well as non- pathogenic groups.
Abbreviations
TCBS: Thiosulphate Citrate Bile Salts Sucrose
SPSS: Statistical Package for Social Science
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!