Biography
Interests
Dr. Okigbo, R. N.1*, Dr. Okigbo, J. E.1 & Dr. Akpaja, E. O.2
1Department of Botany, Nnamdi Azikiwe University, Awka, Nigeria
2Department of Plant Biology and Biotechnology, University of Benin, Benin City, Nigeria
*Correspondence to: Dr. Okigbo, R. N., Department of Botany, Nnamdi Azikiwe University, Awka, Nigeria.
Copyright © 2018 Dr. Okigbo, R. N., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The investigation focused on the chemical components of a newly discovered mushroom (Cantharellus species) found in Ukwa-East, Abia State, Nigeria. Fresh mushrooms of Cantharellus species (Eroumunwene) were harvested from bush around Ohanso in Ukwa-East Local Government Area of Abia State, Nigeria. Mushroom samples were prepared for spore printing which were used in identification and characterization. Samples were brushed to remove soil particles, dirt and were sun-dried which were used in quantitative estimation of the phytochemicals, proximate, minerals and nutrients determination of Cantharellus species (Eroumunwene). Results revealed that the phytochemical constituents of Cantharellus species (Eroumunwene) were Alkaloids (3.73mg/100g), Tannins (0.04mg/100g), Phenols (0.67mg/100g), Saponins (1.21mg/100g), and Flavonoids (7.25mg/100g). The results of proximate composition of Cantharellus species (Eroumunwene) includ 16.92% Protein, 12.33% Fats, 3.75% Fibre, 42.34% Carbohydrate and 9.24% Ash. The nutrient and mineral compositions of Cantharellus species (Eroumunwene) were Nitrate (4.10mg/10g), Nitrite (0.02mg/10g) and Sulphate (0.06mg/10g), Calcium (180.3mgkg-1), Magnesium (84.2mgkg-1), Potassium (202.4mgkg-1), Sodium (53.1mgkg-1), Phosphorus (80.41mgkg-1), Nitrogen (0.82mgkg-1), Iron (321mgkg-1), Zinc (31.2mgkg-1), Copper (10.38mgkg-1) and Manganese (11.53mgkg-1). Heavy metal concentration indicated Nikel (0.33ppm) while Aluminum (Al) and Lead (Pb) were not detected. The results obtained indicate that Cantharellus species are good source of phytochemicals, proximate components and minerals. Consumption of Cantharellus could enhance good health in individuals. Heavy metal levels obtained indicate that the mushrooms are safe for consumption. However, further studies on its polysaccharide contents, toxicity and medicinal value are highly recommended.
Introduction
Mushrooms are macro-fungi with distinctive body, which can be hypogenous or epigenous, and large
enough to be seen with the naked eye and to pick by hand [1]. Also mushrooms are fungi fruit-bodies which
spontaneously appear in forests and farm lands in great quantities after rain [2]. The natural substrata of
mushrooms include logs of wood, decomposing agro-wastes and decomposing animal wastes; also agricultural
waste (oil palm fruit fibre) and topsoil serves as substrate for the cultivation of mushroom where they obtain
their nutrients through external digestion and absorption by the mycelium [3]. Mushrooms have long been
used as a valuable food source and pharmacologically important compounds useful in medicine especially
in traditional medicines around the world, especially in Japan and China, and recently other parts of the
world. Some mushrooms stored glycogen and animal polysaccharide, while others have been implicated in
other biochemical activities [1,2,4]. There are edible and poisonous mushrooms and both categories possess
nutritional and medicinal values. Mushrooms have been reported as therapeutic foods, useful in preventing
diseases such as hypertension, hypercholesterolemia and cancer [2].
World Bank (1992) noted that the widespread of malnutrition with ever increasing protein gap in our country has necessitated the search for alternative sources of protein [5]. This is due to the high population growth, the production of pulses has not kept pace with our requirements, and the animal protein is beyond the reach of most people in this country; owing to the fact that most of the people (over 86%) live below poverty level. Edible mushrooms are recommended by the Food and Agriculture Organization (FAO) as food, contributing to protein nutrition of developing countries dependent largely on cereals [6]. In Nigeria, the rural dwellers consume mushrooms as delicacies in soups and as ingredients for seasoning or part of the local melon cake (a local snack called ‘usu’ in Igbo). For instance, the sclerotia of Pluerotus tuberregium is used as thickener, as well as in preparing melon cake (‘usu’) [2]. Also investigation indicated that mushrooms served as an alternative source of in- come to rural people of Anambra State, Nigeria [3]
The level of mushroom nutriceutricals on a global scale confirms that mushrooms are good health food and information abound in Nigeria on their use for the treatment of malnutrition in infants, diabetes, obesity or hyperlipidemia, sterility, anemia, mumps, fever and protein deficiency improving the treatment of diseases using fungal drugs. Mushrooms have natural antibiotics, triterpenes, glycoproteins and polysaccharides which boost the human immune systems, replace worn out cells and prevent diseases in the body [7-10]. In the 16th century, herbalist John Gerard recommended Auricularia auricula- judae for curing sore throat.
He recommended the preparation of a liquid extract of the mushroom by boiling the fruit bodies in milk, or leaving them steeped in beer, which would then be sipped slowly in order to cure sore throat. It is used as blood tonic, and had been reported that this same Auricularia auricula-judae, was used to cure eye disease and Jaundice when boiled in milk [11-13]. These functional characteristics are mainly due to their chemical composition [12,13].
According to Gucia et al. (2011), edible wild mushrooms contain in the flesh, a spectrum of mineral macro and micronutrients, non-essential trace elements and problematic heavy metals [14]. Organisms require trace amounts of some heavy metals, including iron, cobalt, copper, manganese, chromium and zinc; excessive levels of these metals, however, can be detrimental to organisms [15]. Other heavy metals such as cadmium and lead have no known beneficial effect on organisms [15]. The ability of mushroom species to bio-accumulate minerals from the growth medium into the fruiting body is well documented [16]. The fruiting bodies of higher mushrooms are relatively rich in mineral constituents due to some ecological as well as genetic factors (yet unknown) [17]. Biological factors such as species of mushrooms, morphological part of fruiting body, developmental stages and age of mycelium, biochemical composition and interval between the fructifications affect mineral accumulation in macro fungi [18]. Iron, copper, manganese, zinc (trace elements), lead, cadmium and nickel (toxic metals) were chosen as representatives, whose levels in the environment represent a reliable index of environmental pollution [18]. Minerals such as iron, copper, zinc and manganese are essential metals because they play important role in biological systems, whereas lead and cadmium are non-essential metals as they are toxic, even in traces [19]. The essential metals can also produce toxic effects when the metals intake is excessively elevated [18].
The present investigation focused on the chemical components of a newly discovered mushroom (Cantharellus species) found in Ukwa-East, Abia State.
Materials and Methods
Fresh mushrooms of Cantharellus species (Plate 1A) were harvested from bush around Ohanso in Ukwa-
East Local Government Area of Abia State (Plate 1B). The mushroom was brushed carefully to remove soil
particles and dirt and sun-dried until they become brittle [20].
Harkonen et al., (2015) methods were adopted [21]. Some mushrooms were separated into pileus and stipes.
The pileus was used by placing the gills (fertile side) down on a white paper, covered with bowls to prevent
air currents and left to stand in a corner for 24 hours.
The spore print and wild species of the mushroom (Plate 1C-1E) were sent to Dr. E.O. Akpaja at University
of Benin, Edo State for identification and classification of the mushroom.

The work was carried out at Oshotech Analytical Laboratory, off Omore road, off Ugbowo/Oluku Road,
Benin City. The dried mushrooms were grounded to fine powder using corona blender (Lander). Preservation
of the specimen was done with specimen bottle at room temperature.
Phytochemical determination of the mushroom was carried out using methods from different authors. Each
value is the mean of three replicate determinations ± standard deviation.
One gram of sample was analysed in accordance with the alkaline precipitation gravimetric method with
some modifications [22]. The alkaloid was expressed as percentage.
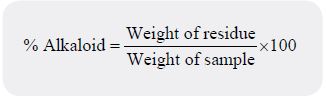
Ethyl acetate precipitation method was used for the determination of flavonoids [23]. One gram of the
sample was hydrolyzed by boiling in 50 millilitres of 2M hydrochloric acid (HCl) solution for about 30
minutes. The hydrolysate was filtered to recover the extract. Five millilitres of the filtrate was pipetted into a
flask and 5 millilitres of ethyl acetate was added starting with a drop to obtain precipitate. The precipitated
flavonoid was recovered by filtration using a weighed filter paper, after drying in the oven at 80°C for 1 hour,
it was cooled in a desiccator and reweighed.
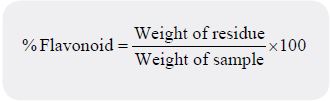
The determination of tannin was done using the Association of Official Analytical Chemists method of
analysis [24]. The mixture of 0.5 gram of sample and 100 millilitres of distilled water was boiled gently on a
hot plate for one hour and filtered. The paper was washed with distilled water and extract diluted to volume,
then cooled. Fifty millilitres of distilled water and 10 millilitres of diluted extract (aliquot volume) was
pipetted into a 100 millilitres conical flask, followed by 5 millilitresFolin-Denis reagent and 10 millilitres
of saturated Na2CO3 solution. The volume was diluted with distilled water. The solution was allowed to
stand for 30 minutes in a water bath (25°C). Optical density was measured at 720nm wavelength and
optical density (absorbance) compared on a standard tannic acid curve. A graph plot of absorbance versus
tannic acid concentration was made with the line passing through the origin. Thus, the tannin content was
calculated as:
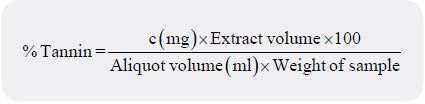
Where C (mg) = Concentration of tannic acid read from the graph
A gravimetric method employing the use of a soxhlet extractor and two different organic solvents was used
[24]. Some quantity of petroleum ether enough to cause a reflux was poured into 1g of sample inside the
soxhlet extractor chamber fitted with a condenser and a flat bottomed flask. The sample was extracted off its
lipid and interfering pigments for 3 hours by heating the flask on a hot plate and the solvent distilled off.
For the second extraction, methanol enough to cause a reflux was poured into the sample. The saponin was
exhaustively extracted for 3 hours by heating the flask on a hot plate; after which the solvent was distilled off.
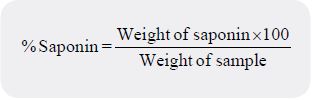
Follins method as described by Pearson (1975) with some modifications was used to determine the phenol
content. Mixture of 0.5 gram of sample and 100 ml of distilled water was boiled for about 30-40 minutes
with constant stirring [25]. It was allowed to cool and filtered into 100 millilitres volumetric flask using
Whatman No 42 filter paper and was brought to mark with distilled water. One millilitre of the filtrate was
treated with 2.5 millilitres of follinciocacteu reagent followed by the addition of 2 millilitres of 2% Na2CO3 solution and was incubated for 15 minutes at room temperature for colour to develop. The absorbance of
the developed colour was measured at 765nm wavelength. Also standard phenol solution was prepared, one
millilitre of the standard solution was treated with follinciocacteu reagent and 2% Na2CO3. The absorbance
was also measured at 765nm wave length and measurement was made with reagent blank at zero. The phenol
content was calculated as:
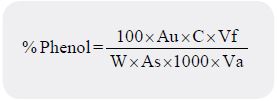
Where W =Weight of Sample analysed, Au = Absorbance of the test sample,
As = Absorbance of standard solution, C = Concentration of standard in mg/ml,
Vf = Total Filtrate volume, Va = Volume of filtrate analysed
Proximate analyses of the mushroom were carried out using Association of Official Analytical Chemists
method [26].
One gram of sample was wrapped properly in a fat- free filter paper (to serve as thimble) and put in a
Sohxlet apparatus or extractor. A weighed flask containing 250cm3 of petroleum ether was placed on a
heating mantle maintained at low temperatures (50 to 70⁰C). As the solvent gets heated up, the hot solvent
rises and drips through the material and in the process extracts the fat which discharges into the flask. The
process was continued until all the fat has been extracted from the material. After extraction, the solvent was
evaporated off the flask. The flask and fat are then left to dry in an oven for 1 to 2 hours. The flask and fat
obtained were then weighed. The crude fat content was expressed as a percentage of the sample used:
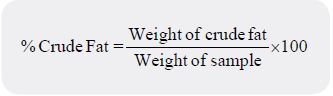
One gram of sample was weighed into a previously weighed Crucible. The crucible + Sample was then put
into a muffle furnace and ignited at 500⁰C for 3 hours until all carbon has been removed. The percentage
ash was calculated as follows:

One gram of the powdered dry sample was weighed in clean dry glass petri dish. The sample was placed
in an electric oven at 105°C and allowed to dry for about 6-8 hours. The oven dried sample was weighed
and placed back in the oven for one hour for further heating. The sample was then weighed again and this
process was repeated until the weight became constant. The percentage moisture content was calculated as
follows:

The percentage dry matter (DM) content was determined as follows:
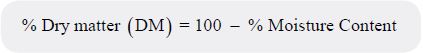
One gram of the sample was placed into 250 millilitres beaker and was hydrolyzed by adding 200 millilitres
of 1.25% Sulphuric acid (H2SO4) and was boiled under control for 30 minutes on a hot plate. The mixture
was filtered while hot through a mulsan filter paper, then rinsed with hot distilled water to neutralize.
The residue was placed back to a beaker with a spatula and boiled with 200 millilitres of 1.25% Sodium
hydroxide (NaOH) for 30 minutes, and was then filtered and rinsed with distilled water. The anti-foaming
agent (vegetable oil) used was 2-4 drops. Ten millilitres of 5% Hydrochloride (HCl) solution was used to
wash the residue and rinsed with hot distilled water to neutralize. Finally, it was rinsed with petroleum ether.
The residue was transferred into a weighed crucible and placed in the oven at 105°C for one hour to dry (the
residue at this stage consists of fibre and ash). The weight of the dried residue and crucible was taken. The
residue was ashed in a muffle furnace at 500°C for 3 hours to burn off the crude fibre. The weight of the fiber
was calculated and expressed as a percentage of crude fiber as follows:
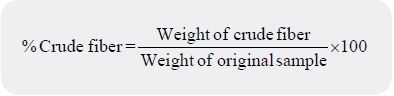
One gram of the sample was placed into a kjedahl flask and 25 millilitres of sulphuric acid (H2SO4) was
added. Three grams of sodium sulphate (Na2SO4) was added to raise the oxidation and boiling point of
the acid. Selenium dioxide was used as a catalyst to speed up digestion. Heat was applied until the mixture
was clear (acid fumes in form of nitrites and nitrous oxides are given off), when all the nitrogen present in
the sample have been converted to ammonia. The colour changed from dark brown to golden yellow. The
flask was allowed to cool, distilled water was added (to make up 100 millilitres mark in a 100 millilitres
standard volumetric flask) to reduce the acidity of the digest. Some quantity (in excess) of 40% sodium
hydroxide (NaOH) was added to the digest and heat was applied, steam produced and ammonia (NH3) gas
was released. This gas was dissolved in the condensing steam as it passed to the water condenser. The gas
was finally trapped in 4% solution of boric acid (H3BO3) to form ammonium borate (NH4BO4). NH4BO4
was titrated against 0.1N dilute HCl. The end point was achieved when the faint green distillate turned to
faint pink colouration. The crude protein content were obtained by multiplying the nitrogen content by a
conversion factor (CF) 6.25. Then the percentage Nitrogen content was calculated and expressed as follows:
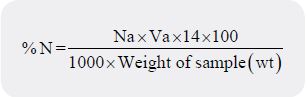
Where Na = Normality or Molarity of acid (HCL), Va =Titre value of acid
14= Atomic weight of Nitrogen
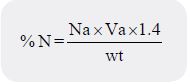
%Crude Protein =%N×C.F
Nitrogen free Extract (NFE) was calculated by difference after analysis of all the other items method in the
proximate analysis.

Mineral contents were determined by Atomic Absorption Spectrometry (AAS) according to the method
of AOAC (2003) while Nitogen (N) was done using micro kjeldahl method of crude protein determination
[26]. The digested sample was analyzed for mineral contents using atomic absorption spectrophotometer
(AAS model Solaar 969, Unicam). The following are the wavelength used for different elements: Copper
(Cu) - 324.8nm, Iron (Fe) - 248.3nm, Zinc (Zn) - 213.0nm, Calcium (Ca) - 422.7nm, Magnesium (Mg)
- 285.2nm, Potassium (K) - 766.5nm, Sodium (Na) - 589.0nm, Manganese (Mn) - 279.5nm, Nikel (Ni) -
232.0nm, Aluminium (Al) 522.0nm, Lead (Pb) - 217.0nm and Phosphorus (P) - 660nm.
Nutrients determination were done by spectro-photometry according to the method of [26].
The 0.1 gram of sample was mixed with 50 millilitres of extracting solution of 0.15% calcium chloride
(CaCl2.2H20) and shook for 30 minutes. The solution was filtered and the absorbance was taken using
spectro-photometer at 520nm wavelength.
The 0.1 gram of sample was put into a test tube and 10 millilitres of de-ionised water was added. The mixture
was heated for one hour at 45°C and it was transferred into a shaking bottle and shook for 15 minutes. It was
allowed to stand and cool for 30 minutes to one hour. The mixture was centrifuge at 5000rpm for 15 minutes.
The supernatant was recovered through Whatman No 42 filter paper into reagent bottle. One millilitre of
supernatant was put in each test tube. One millilitre of stock solutions of Sodium nitrite was added to the
first test tube and potassium nitrate to the second test tube. One millilitre each of 0.5% Sulfanilic acid and
2M of hydrochloric acid solutions were added to each test tube and the mixture were shook thoroughly
for 5 minutes to allow the di-azotization reaction to go to completion. Then one millilitre of 0.5% methyl
anthranilate and 2 millilitres of 2M of Sodium hydroxide were added to each test-tube to form an azo dye
and the contents were diluted to 10 millilitres using water. After dilution, absorbance of each test tube of the
red coloured dye was measured at 470nm wavelength.
Results
The quantity of phytochemicals in Cantharellus species is shown in Table 1. The highest phytochemicals found
in the mushroom was Flavonoids (7.25±0.00mg/100g) while the least was Tannins (0.036±0.00mg/100g).
Table 1 equally showed the proximate analysis of Cantharellus species. Apart from Nitrogen Free Extract
(NFE) that has 42.339±0.064%, protein was the most abundant nutrient with percentage value of
16.916±0.00% and the least occurred in crude fibre with percentage value of 3.75±s0.00%. Equally, the dry
matter content was very high with percentage value of 84.573±0.012%.
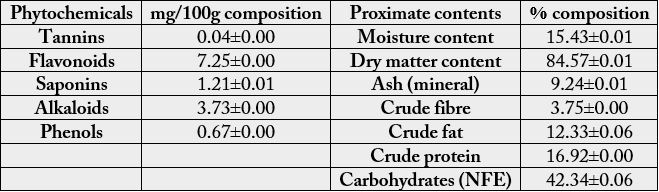
Values are mean of three replicates ± standard deviation.
Mineral composition of the mushroom analysed include the major elements and trace elements. The major elements analysed were potassium (K), sodium (Na), magnesium (Mg), calcium (Ca), phosphorus (P) and nitrogen (N). Potassium was the most abundant major element 202.4±0.00 mgkg-1 found in Cantharellus species, followed by Calcium 180.3±0.00 mgkg-1 while the least was Nitrogen 0.82±0.02 mgkg-1 (Table 2). The trace elements analysed were zinc (Zn), copper (Cu), iron (Fe) and manganese (Mn). Iron was the most abundant trace elements found in the mushroom 321±0.00 mgkg-1 while the least was Copper 10.38±0.01 mgkg-1 (Table 2).
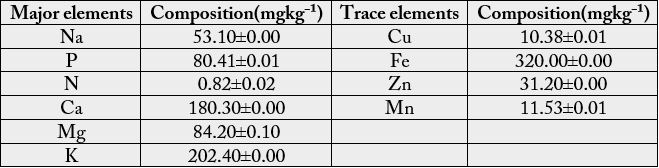
Values are means of three triplicates ± standard deviation
In nutrient analysis, Nitrate (NO₃), Nitrite (NO₂) and Sulphate (SO₄) were analysed. The mushroom has a high content of nitrate 4.10±0.00mg/10g, followed by sulphate 0.06±0.01mg/10g. Then the least was nitrite 0.02±0.01mg/10g (Table 3). Some heavy metals were also analysed, Aluminium (Al) and Lead (Pb) were not detected while Nikel (Ni) was the least detectable element (0.33±0.00ppm) of all the elements analysed (Table 3).
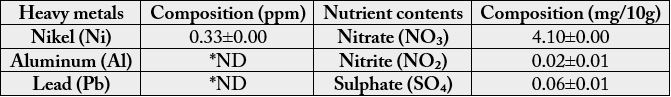
Values are means of three triplicates ± standard deviation.
*ND means Not Detected
Discussions
The results obtained indicate the presence of phytochemicals in varying levels.Flavonoid obtained in this
study was higher than the ones reported in similar study by Okwulehie et al., (2014) on two indigenous
species of mushrooms Russule girolle (Rg) and Daedaleopsis confragosa (Dc), Okwulehie and Ogoke (2013)
on Cheimonophyllum candidissimus,Pleurotus sp., Russula sp. andAuricularia sp. And Kumari et al., (2011)
on three different species of Cantharellus: C. friessi, C. subcibarius, C. cinerius and Pleurotus florida. Equally
alkaloids, saponins and phenol were higher with least amount of tannin when compared with similar works
of Okwulehie et al., (2014) and Okwulehie and Ogoke (2013). These chemicals are considered to be antinutrients
because they have been reported to cause deleterious effects when consumed in large quantities,
however some of them have been shown to have useful application in diets and certain biochemical activities
in animals [20]. So there is need for further research work to ascertain digestibility of these phytochemicals
in mushrooms on human begins when consumed.
The result also showed that the mushroom is good sources of protein and carbohydrate which are of great demand in both man and animals. Protein obtained in this study was higher than the ones reported in similar study by Okwulehie et al., (2014) and Okwulehie and Ogoke (2013). This mushroom is richer source of protein than most commonly consumed vegetables in Nigeria, while the protein content is lower than eggs, meat and fish, it is adequate to be used as an alternative to fish and meat in rural and urban areas were these items are expensive [29]. Although the result showed that it has high content of fat when compared with the works of Okwulehie et al., (2014), Okwulehie and Ogoke (2013) and Ayodele and Okhuoya (2009).
The mushrooms are known to contain varying amount of Calcium, Potassium, Magnesium, Phosphorus, Iron, Zinc, Copper, Manganese and Sodium and these elements are very important in human nutrition. They are required in repairing worn-out cells, strong bone and teeth, building blood cells and maintaining osmotic balance [30]. There is low concentration of heavy metal (Nikel) in the mushroom, Lead and Aluminium were not detected. According to FAO/WHO (1996) standards, these heavy metals are well below the recommended safe values. In other words, Ni, Al and Pb concentrations of the sample were below the tolerance limits established by the FAO/WHO. Hence, this species is recommended for consumption and other uses.
Conclusion
Consumption of mycophagy should be encouraged to promote food security and reduce protein and mineral
deficiencies prevalent in the diet of the people. However, further studies on its polysaccharide contents,
toxicity and pharmacological value are highly recommended.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!