Biography
Interests
Wakshum Shiferaw Gemeda
Arba Minch University, College of Agricultural Sciences, Natural Resources Management, Arba Minch, Ethiopia
*Correspondence to: Dr. Wakshum Shiferaw Gemeda, Arba Minch University, College of Agricultural Sciences, Natural Resources Management, Arba Minch, Ethiopia.
Copyright © 2019 Dr. Wakshum Shiferaw Gemeda. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This paper was aims to assess the effects of Prosopis juliflora (Sw.) DC (P.juliflora) on the abundance, composition, and activity of soil microbial and pathogenic communities, and their effects soil communities of beneficiary plant species. P.juliflora is effective in increasing the total mycorrhizal colonization of roots and reducing heavy metals such as Cadmium (Cd) levels in plants. Results depicted that intensity of mycorrhization of P.juliflora was significantly higher than that of native Acacia species. On the other hand, in study results in all sites in Saudi Arabia revealed that Cmic of P.juliflora was greater in the rhizosphere of P.juliflora than in the rhizosphere of Acacia ehrenbergiana and Acacia tortilis. Extracts from parts of P.juliflora are used in disinfecting, and also potentials in various bio-functions against different bacterial pathogens. These extracts are more active in green leaves than dry leaves. The diverse soil microbial associations, antagonistic and biopharmaceutical functions of the extracts of P.juliflora might change the negative perceptions of communities using it and impacts on native plant species.
Introduction
P.juliflora belongs to the family Fabaceae (Leguminosae), subfamily Mimosoideae and genus Prosopis. It
is a well-adopted shrub to harsh environmental conditions of many arid zones [1]. P.juliflora is originated
from South America, the Caribbean, and Central America [2]. The species is distributed in the lowlands of
Americas, Africa and Asia [3].
The ecology or habitats of P.juliflora are grasslands, shrubland, and dry forests. This is a salt and drought tolerant tree/shrub species. Moreover, it is nitrogen-fixing, has deep-reaching roots and tolerates dry as well as waterlogged soils [4]. P.juliflora is found in areas where water and poor soil fertility are the principal agents limiting plant growth and can survive and even flourish on some of the poorest land, unsuitable for any other tree species [3]. However, the impact of plant invasions on ecosystems, habitats and native species is severe and often irreversible [5].
P.juliflora is one of the most economically and ecologically important tree species in arid and semi-arid zones of the world. It is an important species because of its high nitrogen-fixing potential in very dry areas and drought seasons [6]. Moreover, P.juliflora is effective in increasing the total mycorrhizal colonization of roots and reducing heavy metals such as Cadmium (Cd) levels in plants. This is affected by Coir pith that is a byproduct of the coir industry and P.juliflora charcoal prepared by burning P.juliflora plant wood [7].
The most probable number analysis showed that density of rhizobia organisms which were able to nodulate P.juliflora was greater than for Acacia species. Arbuscular mycorrhizal fungi colonization in the roots of native species was also affected by P.juliflora [5].
P.juliflora is among nurse plants acting as facilitation and involved in the organization of plant communities and maintenance of biodiversity, particularly in harsh environments. Nurse plants increase plant diversity and productivity in these ecosystems, but our knowledge on the mechanisms through which such facilitation operates is still expanding. Despite of the growing evidence of soil microbiota impacts on plant fitness and plant community dynamics, facilitation of nurse plants in terms of microbial associations were less understood [8].
Globally, several types of research were conducted regarding the effects of P.juliflora on native species, composition, and diversity [9,10], its effects on soil properties [11,12], allelophystic effects on associated plants [13], impacts on socioeconomic activities [2,14,15], but its role in terms of plant facilitation has been little explored. Thus, this paper tried to assess the effects of P.juliflora on the abundance, composition, and activity of soil microbial and pathogenic communities, and their effect of these soil communities on beneficiary plant species.
Materials and Methods
To compile this paper, 27 selected papers such as books, research papers, manuals, reports, and proceedings
were reviewed. Main findings of research papers and textbook facts are tried to verify and reviewed to
achieve the objectives of the paper.
Results
In arid and semi-arid systems results show that nurse plants promote the development of differentiated
soil microbial communities characterized by a higher microbial abundance and activity, the dominance of
competitive bacteria and larger mycorrhizal networks, compared to coexisting non-nurses [8]. Microbial
associated plants enhance and facilitate the growth of other plants (target species). Plant-plant facilitation is
thus a process by which one plant species has a positive effect on the germination, survival growth or fitness
of other species [16-18]. In this paper, P.juliflora can be taken as nurse plants for other native species.
According to McIntire and Fajardo [18], biotic drivers in this case plants create and maintain biological diversity within trophic levels have focused primarily on negative interactions (i.e. competition), leaving marginal room for positive interactions (i.e. facilitation). However, understandings of plant ecology have an aboveground bias that neglects soil micro-organisms in plant species interaction environment [17].
Significance of Microbial Mechanisms of Plant-Plant Interactions
Communication among organisms is one of the best gifts of nature that played a key role in the evolution
and involvedness of life on Earth [19]. For instance, one symbiont might be a better competitor for soil
resources and might more efficiently deliver these remunerations to a single host species. This would be
consistent with the competitive dominance of the host species. On the other hand, plant-plant interactions
could be stabilized by negative feedback generated by highly disproportionate fitness relations between the
plant and symbionts. Such unequal fitness relations resulting in negative feedback have been observed in
interactions between co-occurring plants and arbuscular mycorrhizal fungi [20].
Effects of P.Juliflora Invasion Microbial Colonization
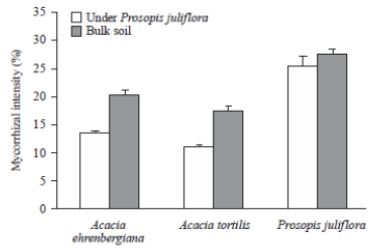
Results depicted that intensity of mycorrhization of P.juliflora was significantly higher than that of native
Acacia species (Fig 1). Moreover, the intensity of mycorrhization of A. ehrenbergiana and A. tortilis decrease
significantly from 20 and 17% (for plant growing on bulk soil) to 13.7 and 11.1%, respectively, for plant
growing on soil collected from under P. juliflora tree canopies [5].
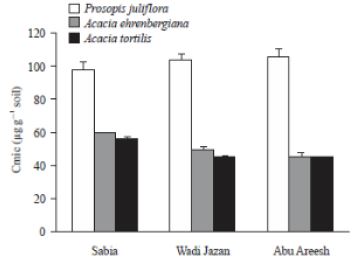
On the other hand, microbial biomass also differed significantly between soils from under P.juliflora and native Acacia species (Fig 2). In all sites, Cmic was greater in the rhizosphere of P.juliflora than in the rhizosphere of A. ehrenbergiana and A. tortilis ([5]).
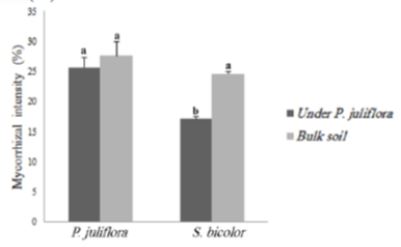
Mahdi et al. [21] reported that P.juliflora negatively affects the arbuscular mycorrhizal fungi colonization of Sorghum bicolor roots. It was found that P.juliflora stimulated the soil microbial biomass carbon, soil metabolic quotient, and soil enzymes activities. For instance, the intensity of mycorrhization in S. bicolor was significantly lowered by 24.6% (plants growing on bulk soil) to 17.1% in plants growing on soil collected from under P.juliflora tree canopies (Fig 3).
Antagonistic and Biopharmaceutical Effects of P.juliflora
It was indicated that P.juliflora had adverse effects on the growth of associated plants namely grass species in
an ecosystem (e.g., Cenchrus ciliaris, Panicum antidotale and Panicum maximum). These outcomes suggest
that P.juliflora foliage contains allelochemicals that had an inhibitory effect on plant growth [22]. Furthermore,
results showed that different aqueous extracts of P.juliflora significantly affected the germination of native
Acacia species. Plant growth of these species was also affected by the litter of the invasive plant.
Results on other aspects revealed that green leaves extract of P.juliflora has a higher zone of inhibition compared with dry leaves with a diameter ranged between 10 and 22 against all tested bacteria and with more inhibition to Gram positives than Gram-negatives (Saadoun et al., 2014). Moreover, Odhiambo et al. [23] reported that ethanolic extract of root and leaves of P.juliflora possessed saponins, tannins, and alkaloids; phytochemicals whose antimicrobial properties were well documented and therefore could be attributed to antibacterial activity exhibited by these extracts. Results from this study strongly validated the use of P.juliflora in the management of bacterial infections.
Components of P.juliflora such as flavonoids, tannins, alkaloids, quinones, or phenolic compounds demonstrate potentials in various bio-functions, such as analgesic, anthelmintic, antibiotic, antiemetic, microbial antioxidant, antimalarial, antiprotozoal, antipustule, and antiulcer activities. Its components also used in the enhancement of H+, K+, ATPases; oral disinfection; and probiotic and nutritional effects; as well as in other biopharmaceutical applications, such as binding abilities for tablet production [24]. On the other hand, P.juliflora contains 24-methylencycloartan-3-one, which can safely be used to treat diabetes mellitus instead of using insulin [25]. This compound is contained in the oil of P.juliflora pods. Additionally, it has a good hypoglycemic effect in the screening assay of alloxan inducing fasted diabetic rabbits. The compound 24-methylencycloartan-3-one shows no cytotoxicity to red mammalian blood cells [26,27].
Conclusion
In several research findings, it was found that abundances and composition of both Rhizobium and
mycorrhizal soil micro-organisms are greater in bulk soils under P.juliflora than under other native species.
These indicated that more bacterial biomass under the soils of P.juliflora is far greater under soils of P.juliflora
than other species, for instance, Acacia species. On the other hand, extracts from parts of P.juliflora are
used in disinfecting, and also potentials in various bio-functions against different bacterial pathogens. These
extracts are more active in green leaves than dry leaves. However, several research reports indicated that the
negative effects of P.juliflora were outwaited that of positive ones. To reverse these problems, more researches
will be carried out to promote and reduce negative integration of P.juliflora with associated native plants.
Conflicts of Interests
The article should be free from any such conflicts between authors or with others in any aspect.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!