Biography
Interests
San San Aye1* & Han Naung Tun2,3
1Pro-Rector, Mawlamyine University, Mon State, Myanmar
2Clinical Medicine, Pun Hlaing Siloam Hospital, Yangon, Myanmar
3Clinical and Research Working Groups, European Society of Cardiology, France
*Correspondence to: Dr. San San Aye, Pro-Rector, Mawlamyine University, Mon State, Myanmar.
Copyright © 2019 Dr. San San Aye, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Medicinal plant Euphorbia hypericifolia L. reputed to possess the curative power in enteric infections was selected, tested and evaluated for their activities by in vitro technique. Euphorbia hypericifolia L. was extracted with 50% ethanol, 95% ethanol, ethyl acetate and aqueous solutions and extracts were used to screen enteric infections. These extracts were found to be potent. Antibacterial activities of its plant was tested, According to the results, the extracts made by organic solvents revealed the distinct antibacterial activity on gram-negative bacteria such as E.coli, Salmonella, Shigella, and Vibrio. It may be used as a remedy for the treatment of dysenteric patients but we need large randomized control trials to confirm its effect in human model.
Introduction
Euphorbia hypericifolia L. belongs to the family Euphorbiaceae. Euphorbiaceae is a large family of 283
genera with about 7300 species, of almost cosmopolitan distribution (Laurence, 1969), mainly tropical [1] but extending also to temperate regions of both the Northern and Southern hemispheres [2]. The
outstanding features of the family Euphorbiaceae are the presence of latex, conspicuous unisexual flowers
and tricarpellary the subtropical and warm temperate regions. It grows wide abundantly in Myanmar. In the
British Pharmaceutical Codex (1954) Euphorbia hypericifolia L. is said to contain water soluble constituents,
gallic acid, quercetin, phenolic substances, an amorphous glycoside and a sugar. The portion soluble in
alcohol, but insoluble in water, contains a monohydric alcohol, euphosterol, a phytosterol and phytosterolin,
jambulol, melissic acid and a mixture of fatty acid [2]. The leaves are eaten as a vegetable and contain 78.14%
moisture, protein, 4.65%; ash 3.15% and vitamin C, 44.32mg/100g. The latex of this plant is used as an
application for warts.
Matindale (1967) stated that a tincture of Euphorbia hypericifolia L. has been recommended since 1932 in the treatment of cough and asthma. In British Herbal Pharmacopoeia (1976), it is prescribed for the treatment of asthma, bronchitis, upper respiratory catarrh, and intestinal amoebiasis. In Malaysia, E. hypericifolia L. has various medicinal uses [3]. The latex is used as eye drop for conjunctivitis and complaints of the eye. It is also used for poulticing sores on the legs and for bruises, and wounds due to the marine worm and cholera. The Philipinos also use it as a poultice. Chinese herbalists also use Euphorbia hypericifolia L. Bacteria are prokaryotic cells whose single chromosome is not contained within a nuclear membrane. The bacterial cytoplasm is surrounded by a plasma membrane (Nicklin, Graeme-Cook, Paget & Killington, 1999). Some bacteria are variable in shape and are said to be pleomorphic (of many shapes). The shape of a bacterium is determined by its rigid cell wall. The size of bacteria is in the range from 0.2-50urn. They show little structural differentiation when examined by ordinary microscopic methods, but special staining method show that they possess a central nuclear body which contains deoxyribonucleic acid and divides by simple fission without evidence of mitosis or chromosomes. Types of bacterial organism were expressed in Table 1.
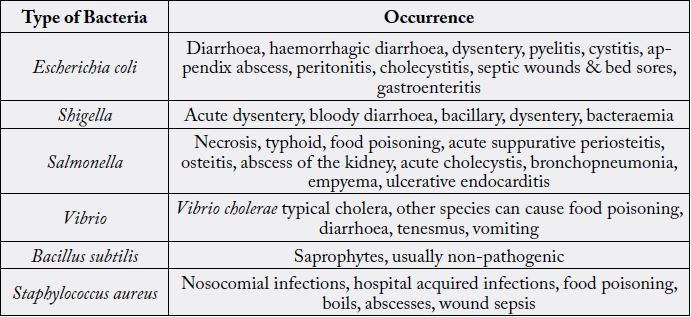
Materials and Methods
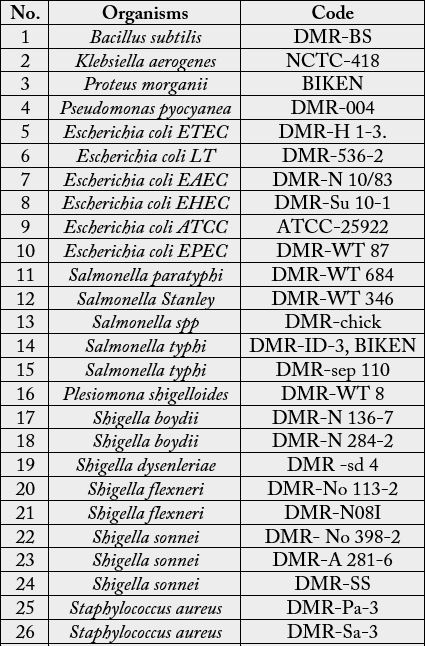
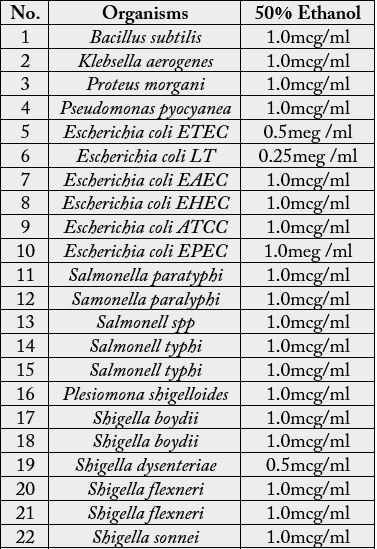
The bacteria species used were obtained from the Department of Medical Research (Lower Myanmar),
Yangon, and listed in Table 1.
Trypticase soy broth from Difco, trypticase soy agar from Becton, and triple sugar iron agar from Difco,
Ethyl acetate, 95% ethanol, 50% ethanol, and distilled water were used. The chemicals used were from British
Drug House (B.D.H Chemical Co. Ltd., (England), Kanto Chemical Ltd., (Japan) and Merck Co., Ltd.,
(Germany). Most of the chemicals and reagents employed in this study were from B.D.H Chemical Co.
Ltd., England", Sigma Chemicals Co., St. Louis, U.S.A", Difco Laboratories, (Detroit, U.S.A), E. Merck,
(Darmstadt, Germany) and Becton Dickson and Co., (Cockeysville, U.S.A).
Conical flasks, petridishes, test tubes, measuring cylinders, a constant temperature bath (Yamato Scientific
Co., Ltd. Japan), a hot air sterilizer (Tomy seiko Co., Ltd., Tokyo, Japan), a refrigerator (National Co., Ltd.,
Japan), Wire loops, straight wire, spirit burner and aluminium foil were employed.
The air dried fine powder (100)g was soaked in 500ml of 50% ethanol, 95% ethanol, ethyl acetate and
distilled water for about 2 days and then filtered. By using a rotary evaporator the solvents were then allowed
to evaporate so as to obtain a paste or powder form. Then the dried extracts were weighed.
For the determination of antibacterial activity of crude extracts in vitro, agar disc diffusion method was used
because of its simplicity, speed of performance, economy and reproducibility.
Trypticase soy agar (40)g was suspended in 1000ml of distilled water in a sterile conical flask and covered
with aluminium foil. Then the suspension was mixed thoroughly and heated completely to dissolve the
powder on a hot plate stirrer [4]. This Trypticase was then sterilized in an autoclave at 121°C for 15mins.
The temperature of the agar solution was reduced to 50°C in a constant temperature bath. Trypticase soy
agar was then poured into sterile petridishes near a flame of a spirit burner. The agar medium was allowed
to solidify and sealed tightly in a polythene plastic bag. The medium was stored in a refrigerator until used.
The solidified agar medium was dried in 42°C incubator before it was used.
Triple sugar iron agar (65g) was suspended in 1000ml of distilled water in a sterile conical flask, covered
with aluminium foil and mixed thoroughly and heated completely to dissolve the powder on a hot plate
stirrer. Then triple sugar iron agar solution was transferred into test tubes (4ml for each) and sterilized
by autoclaving at 121°C for 15mins. After sterilization, the test tubes were placed in a slant position and
allowed to solidify.
Trypticase soy broth (30g) was suspended in 1000ml of distilled water in a conical flask, covered with
aluminium foil, mixed thoroughly and heated on a hot plate with magnetic stirrer till dissolved [4,5]. The
broth solution was then transferred into test tubes (3ml in each tube) and sterilized by autoclaving for
15mins at 121°C.
The bacteria species used were obtained from the Department of Medical Research (Lower Myanmar),
Yangon, and were listed in Table. A few colonies of the organism to be tested were inoculated into the triple
sugar iron agar and incubated at 37°C for 24hrs in an incubator. A few colonies of the organism from triple
sugar iron agar were introduced into the trypticase soy broth and incubated for 3hrs at 37°C to obtain the
bacterial suspension of moderate cloudiness. This contained approximately 105 to 107 organisms per ml.
Water, 50% ethanol, 95% ethanol and ethyl acetate extracts of Euphorbia hypericifolia L. were prepared. The
bacteria species tested were the same as those given in Table 2.
The plant extract (0.2g each) was introduced into sterile petri-dishes and dissolved with 1ml of their respective
solvents viz., 50% ethanol, 95% ethanol, ethyl acetate and distilled water. The antibacterial activity of the
extracts was determined by the agar disc diffusion technique. Screening was done by the use of impregnated
paper-discs (8mm) which were sterilized by autoclaving, followed by dry heat at 60°C for 1hr. It was then
impregnated with concentrated extracts and allowed to dry at 42°C in an air flow incubator. The bacterial
suspension from Trypticase soy broth was streaked evenly onto the surface of the Trypticase soy agar plates
with a sterile cotton swab (Puriton, USA). After the inoculum had dried (5mins), the dried discs were placed
on the agar with flamed forceps, gently pressed down to ensure proper contact. A disc impregnated with
solvent only was placed along the side of test discs for control and comparing purposes. The plates were incubated immediately (or within 30mins) after inoculation. After overnight incubation at
37°C, the zones of inhibition diameter (including the 8mm discs) were measured.
In order to determine the minimum concentration of extracts that inhibit the microorganism, the specific
concentrations of extracts prepared in serial dilution in agar plates were used for testing with microorganisms.
Fifty percent ethanol extract of Euphorbia hypericifolia L. was selected for determination of MIC. The
minimum inhibitory concentration (MIC) of the extracts was determined by test tube serial dilution method
[4,5].
The active plant extracts were dissolved with their respective solvents (e.g., ethanolic extracts with ethanol) and diluted with trypticase agar to obtain the following concentrations (e.g. 0.5g) of the plant extracts was dissolved in 2ml of solvent and diluted with 40ml of trypticase soy agar, where the concentration will be from MIC 0.625mcg/ml to 1mcg/ml. The above solutions of varying concentrations were each poured into sterile petridishes. Trypticase soy agar containing only the solvent was also prepared with sterile petridish for control purposes. Then the agar medium were allowed to solidify.
Bacterial suspension was obtained by inoculation of a few colonies of the organisms to trypticase soy broth and incubated at 37°C for 3hrs. The bacterial suspension was streaked onto the surface of the prepared agar plates. Then the plates were incubated at 37°C overnight. After this, the lowest concentration showing no growth of the organisms was taken as the minimum inhibitory concentration (MIC) expressed in mg/ml. The experiments were repeated three times at exactly the same parameters, and the mean results were taken.
Results
The antibacterial activity of Euphorbia hypericifolia L. extracts by different solvents were estimated by agar
disc diffusion method of Ananthanaragan & Paniker (1983). In this test 35 species of bacteria, including
eight species of Shigella, eight species of Staphylococcus aureus, six species of Escherichia coli, five species of
Salmonella, three species of Vibrio, a species each of Bacillus subtilis, Klebsiella aerogenes, Proteus morganii and
Pseudomonas pyocyanea, were used as test organisms (Table 3).
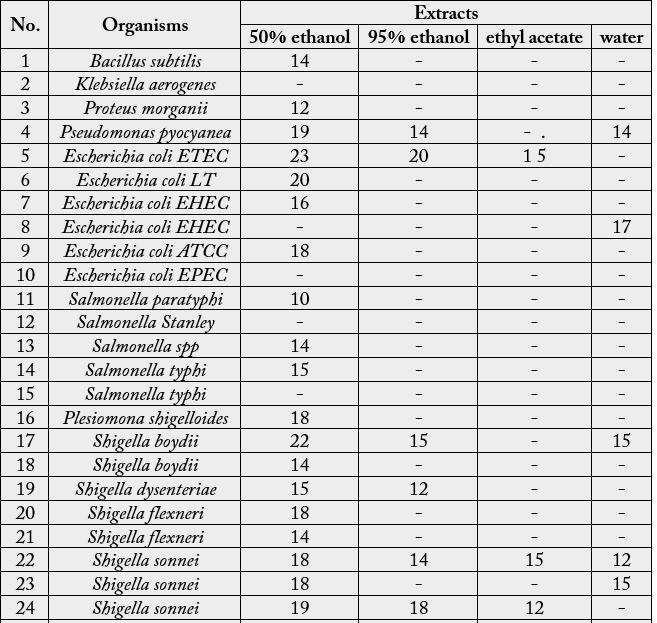
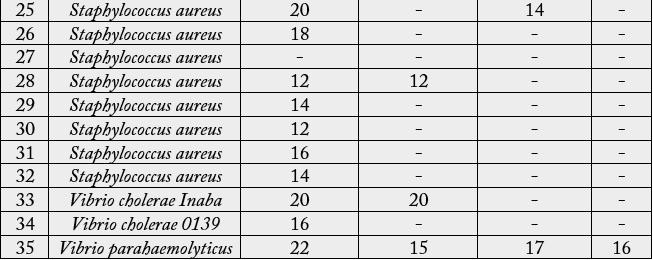
The antibacterial activity revealed by different extracts of Euphorbia hypericifolia L. has been shown in Table 3, comparatively as the zone of inhibition in mm affected by different extracts and also on respective test organisms. Four different types of Euphorbia hypericifolia L. extracts were tested on the 35 species of bacteria. The extracts using 50% ethanol were the most effective and these exhibited antibacterial activity on 29 of the total 35 species tested. Extracts with 95% ethanol showed activity against 9 species, while the extract with ethyl acetate showed antibacterial activity on only 5 species' while that with aqueous solution showed antibacterial activity on only 6 species. It has been noted that the 50% ethanol extract showed the maximum inhibitory zone of 23mm on E. coli ETEC and 22mm on Vibrio parahaemolyticus (Plate c and d). But it showed minimum antibacterial activity (12mm) on Proteus morganii and Staphylococcus aureus.
The 95% ethanol extract of Euphorbia hypericifolia also revealed the moderate antibacterial activity of 20mm inhibitory zone on E. coli LT as shown in the case of 50% ethanol extract. It also showed the similar effect of Vibrio cholerae Inaba (20mm) zone has been shown on Shigella dysenteriae (12mm with 95%) and Staphylococcus aureus (20mm with 95%). When Euphorbia hypericifolia L. was extracted by ethyl acetate, less amount of inhibitory zone (17 mm) on Vibrio parahaemolyticus 15mm on E. coli ETEC and Shigella sonnei) occurred. Moreover, the aqueous extract of the plant also gave the (12 to 17mm) zone on E.coli, Vibro, Shigella and Pseudomonas pyocyanea (Plate 2. a).
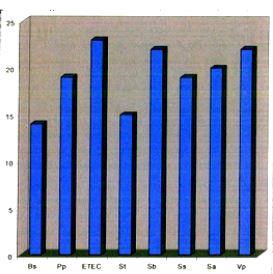
Bs = Bacillus subuls Sb = Shgella boydii
Pp = Pseudomonas pyocyunea Ss = Shigella somei
ETEC = Enterotoxigenic Escherichia coli
Sa = Staphyl ococcus aureus
St = Salmonella typht Vp= Vigrioparahaemolyticus
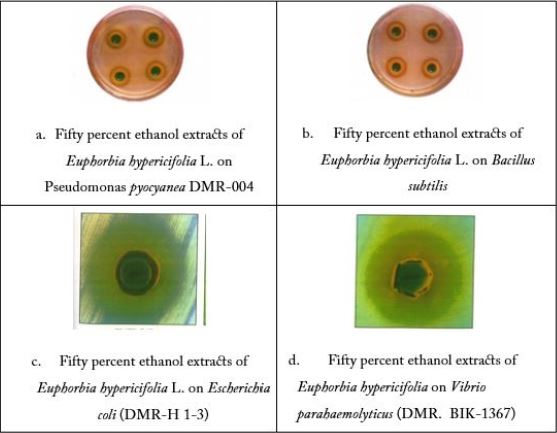
It was clear that the screening of antibaterial activity by E. hypericifolia L. plant extracts of 50% ethanol, 95% ethanol as well as of ethyl acetate showed the prominent antibacterial activity on E. coli (ETEC), Shigella sonnei & Vibrio parahaemolyticus. Based on the antibacterial activity results, the 50% extract by ethanol was selected for further investigation.
The bacteriostatic and bactericidal activities of the extracts were tested by test tube serial dilution method and
determined the turbidity and growth on the nutrient agar [4]. All the tested extracts showed the bactericidal
activity which coincide with the MIC values. The minimum inhibitory concentration (MIC) of antibacterial
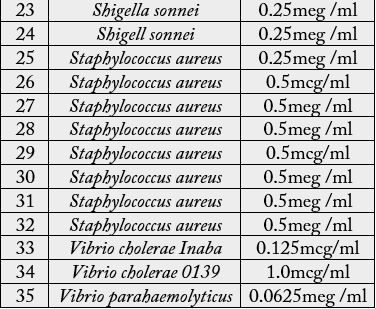
activity 50% ethanolic extracts of Euphorbia hypericifolia was shown in Table 4. All the test organisms were
the same as the former experiments, MIC ranged from 0.0625mcg/ml to 1mcg/ml with variation on the
type of the bacteria used. The least MIC, 0.0625mcg/ml, was obtained. Finally it has been concluded that
the extracts by organic solvents revealed the distinct antibacterial activity on a gram negative bacteria such
as E. coli, Salmonella, Shigella and Vibrio.
The antibacterial activity of the crude extracts of Euphrbia hypericifolia L, was screened by in on 35 bacteria,
which include Bacillus subtilis, Klebsiella aerogenes, Proteus morganii, Pseudomonas pyocyanea, Escher ichia coli,
Salmonella, Shigella, Stapyllococcus aureus Vibrio [1].
Euphorbia hypericifoLia L. extract has a definite antibacterial activity against dysentery, MIC for 50% ethanol extracts against bacteria was determined as 0.0625mcg/ml. The results show that, 95% ethanol extract has been found to have the best inhibition on the in vitro growth of Entamoeba histolytica, in monoxenic culture. Fifty percent ethanol extract and aqueous extract also exhibited growth inhibition although to lesser degrees.
Discussion and Conclusion
According to the results, the extracts made by organic solvents revealed the distinct antibacterial activity on
gram-negative bacteria such as E.coli, Salmonella, Shigella, and Vibrio. It will be much beneficial, if one can
produce 50% ethanol extract of Euphorbia hypericifolia may be used as a remedy for the treatment of dysenteric
patients but we need large randomized control trials to confirm its effect in human model. It was generally
found that Euphorbia hypericifolia L. had a definite amoebicidal action. It was also confirmed by the recent
discovery of its amoebicidal action by Professor J. David Phillipson who is the present Head of Department
and Professor of Pharmacognosy in the school of Pharmacy, University of London [6]. According to Ridet
and Chartol (1964), Professor Phillipson (1991), there is no toxic action found in Euphorbia hypericifolia L.
It may therefore be of therapeutic importance on both infants and adults. Moreover, Euphorbia hypericifolia
L. has long been a popular folk medicine and is also a known and accepted medicinal plant by the people of
Myanmar [6,7].
Conflict of Interest
I declare there is no conflict of interest in this research.
Acknowledgment
I would like to express my sincere thanks to Bacteriology Research division, Department of Medical Research
(Lower Myanmar) supported the full of facilities of this research. I also deeply thanks to Dr Han Naung
Tun, to publish and edit this paper for his guidance.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!