Biography
Interests
Kingsley Ekwemalor1, Emmanuel Asiamah1, Eboghoye Eluka-Okoludoh1, Bharath Mulakala2, Sarah Adjei-Fremah2 & Mulumebet Worku2*
1Department of Applied Sciences and Technology, North Carolina A&T State University, USA
2Department of Animal Sciences, North Carolina A&T State University, USA
*Correspondence to: Dr. Mulumebet Worku, Department of Animal Sciences, North Carolina A&T State University, USA.
Copyright © 2018 Dr. Mulumebet Worku, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Many factors influence the gut microbiome. The gastrointestinal tract of goats is inhabited by diverse and complex microbial communities including bacteria, protozoa, fungi, archaea, and viruses. This study investigated the shifts in the bacterial community during the periparturient period. Fecal samples were collected from Five BoerXSpanish goats at 14 days and 7 days before and after parturition. Fecal DNA was isolated using the QIAamp (R) DNA isolation stool mini kit. The Nanodrop spectrophotometer was used to determine the concentration and purity of microbial DNA. Fecal samples were amplified using RT-PCR to determine the presence of total microbial DNA and relative abundance of Bifidobacteria spp and Lactobacillus spp. The housekeeping genes GAPDH and β-actin were used to normalize the data. Relative abundance was calculated using the Livak method were samples taken from 2 weeks before kidding served as the control group. Bifidobacteria, Lactobacillus, and 16S primers detected microbial DNA in fecal samples. There was an increase in Bifidobacteria, and Lactobacillus 7 days before kidding. Gut microbial diversity changes in periparturient goats.
Shifts in ruminal bacteria are considered to be beneficial to feed efficiency during the peripartal period [1].
The periparturient period is defined as the period from 3 weeks prepartum to 3 weeks postpartum [2,3].
Immunomodulatory properties of Bifidobacterium and the mechanisms and molecular players underlying
these processes have implications for animal health [4,5]. The gastrointestinal tract of goats is colonized by a
complex microbial community. Diverse microbiota such as bacteria, archaea, protozoa, and fungi play a role in
the host’s nutrient uptake and energy metabolism in ruminants [6]. Lactobacillus is a gram-positive bacterial
species inhabiting the gastrointestinal tract of vertebrates [7]. Although greatly outnumbered by anaerobic
bacterial species in the intestinal tract, lactobacilli are often detected in fecal samples [8]. Bifidobacterium
is among the first microbes to colonize the gastrointestinal tract and are believed to exert positive health
benefits on their host [9]. Previous studies have shown their use as probiotics especially in dairy products
[10,11].
Lactobacillus and Bifidobacterium, are commonly used as probiotics in functional foods and animal feed [12,13]. These species have been shown to protect against enteric infection [4]. The objective of this study was to evaluate the shifts in the bacterial community in goat feces during the periparturient period.
Materials and Methods
Five female BoerXSpanish goats were used from North Carolina Agricultural and Technical State University
Farm according to the guiding principles for the Institutional Animal Care and Use Committee (IACUC
ID: 15-006.0).
Fecal samples were collected and evaluated once a week throughout the experiment.
The QIAamp (R) DNA isolation stool mini kit (QIAGEN Sciences, Maryland) was used to isolate DNA
from fecal samples as recommended by the manufacturer. The concentration (260nm) and quality or purity
(260/280nm) of the isolated DNA samples were determined using the Nanodrop Spectrophotometer 1000
3.7.1 (Thermo Scientific Inc., MA).
DNA isolated from the fecal samples was amplified using PCR to determine the presence of total microbial
DNA and relative abundance of Bifidobacteria spp and Lactobacillus spp. The GAPDH gene was used as a
housekeeping gene and for normalizing data. Specific primers for the amplification of variable regions of
16S rRNA gene for Bifidobacteria spp and Lactobacillus spp were used (Table 1).
PCR was done using the CFX Connect Real-time system (Bio-Rad Laboratories, Inc., USA) [14]. Amplification consisted of one cycle of 95ºC (10 minutes), 40 cycles of denaturation at 95ºC (15s) and annealing/extension at 60ºC (1minute) [15,16] in the CFX Connect Real-time system (BIO-RAD Laboratories, Hercules, CA)
Results
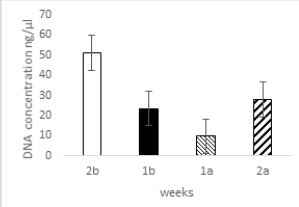
The lowest concentration of DNA in goat fecal samples was isolated during the periods one week before
and one week after kidding. The concentration of isolated DNA ranged from 9.8ng/μl to 51ng/μl. The
highest concentration was observed 2-weeks before kidding. The lowest concentration was observed 1-week
after kidding.
The relative abundance of total microbe (16S), Bifidobacterium spp and Lactobacillus spp changed during the
periparturient period. The relative abundance was high for Bifidobacterium spp and Lactobacillus spp 7 days
before kidding.
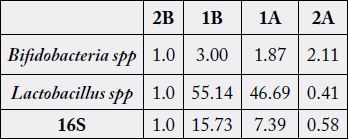
A – After, B – Before
Discussion
Understanding the shifts in bacterial communities in goats during the periparturient period is very
important. The gastrointestinal tract is colonized by a diverse array of microflora which may be detrimental
or beneficial to the host. Bifidobacteria and lactobacillus bacteria have been found in ruminant fecal samples
and used as probiotics for improved production and have also been used to monitor food safety for mutton
and other products [17]. In our study, both Bifidobacteria and Lactobacillus were present in goat feces, and
their abundance varied during the periparturient period. Previous studies conducted by [14] reported the
expression of both Bifidobacteria and Lactobacillus in sheep during the periparturient period. This result
corroborates with the result from our study.
Our results also show an increased population of Bifidobacteria and Lactobacillus 7 days before kidding. It has been shown that oral administration of Lactobacillus casei activated immune cells of the innate immune response and increased the expression of innate immune receptor, TLR2 [21]. In ruminants, the probiotic Lactobacillus rhamnosus have been shown to amend E. coli induced inflammation in primary bovine mammary epithelial cells. Results from this study may suggest the need to study the role of shifts in Bifidobacteria and lactobacillus on host health and well-being during the peripartal period.
Conclusion
Both Bifidobacteria and Lactobacillus were present in goat feces, and their abundance varied during the
periparturient period. Further studies are needed to determine the association to innate immunity during
this period. Detection of fecal microbes in goats may be affected by the period of sampling.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!