Biography
Interests
Anubha Bajaj
Department of Histopathology, A.B. Diagnostics, India
*Correspondence to: Dr. Anubha Bajaj, Department of Histopathology, A.B. Diagnostics, India.
Copyright © 2018 Dr. Anubha Bajaj. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Necrotising enterocolitis is an intestinal inflammatory disorder predominantly affecting the preterm. The deterioration ranges from minimal mucosal trauma to pervasive necrosis of the bowel wall with perforation. The condition is an essential cause of neonatal mortality particularly the very low birth weight (VLBW) [1]. Considerable morbidity in the neonatal intensive care is delineated with necrotising enterocolitis despite early detection, diligent therapies and ameliorated consequences.
Prevalence
Necrotising enterocolitis is evidenced in up to 13% infants of ≤ 33weeks gestation or with a birth weight of < 2500gm [1,2,3]. Neonates at term or ≥ 35 weeks of gestation display enterocolitis like gastrointestinal symptoms accompanied by disorders such as congenital heart disease, perinatal asphyxia, polycythaemia, sepsis and respiratory disease.
Pathogenesis
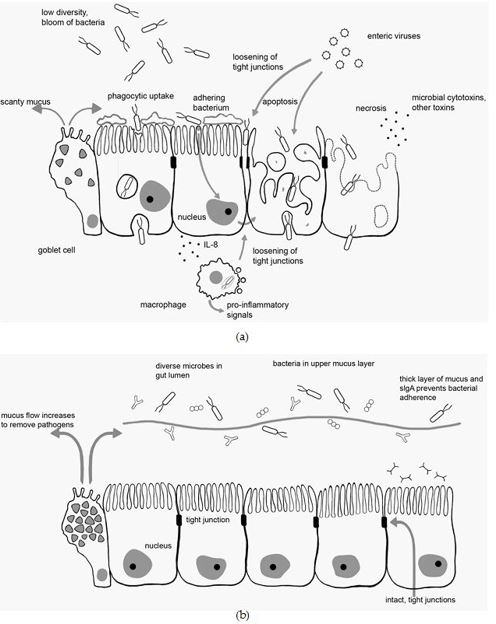
The hyperactive environment of the premature intestine, central feeding and intestinal bacterial flora determine the progress of the disease. The preterm and full term intestinal tract are markedly dissimilar in the bacterial colonization, microcirculation, perfusion, maturation of the inherent gastrointestinal immune system etc, thereby describing the multi- factorial pathogenesis of necrotising enterocolitis. The Toll like receptor 4 (TLR4) is reinforced in the preterm, consequent to the upgraded TLR4 gene which modulates the normal gastrointestinal tract.
The TLR4 levels impact the preterm intestinal substratum along with the gram negative bacteria colonizing the gastrointestinal tract The preterm intestine with the intense TLR4 levels induces the enhanced enterocyte apoptosis, diminished mucosal healing with an intense pro-inflammatory cytokine release to activate the inflammation [4]. The gastrointestinal mucosal gram positive bacteria initiate the TLR4 on the immature bowel mesentery endothelium to diminish the blood flow and provoke intestinal ischemia and necrosis. This mechanism of inflammation is designated as “the cross switching hypothesis “. It presumes that the infection in the premature is secondary to bacterial colonization. The distinction between the preterm and full term host contributes as
These factors contribute to the pro-inflammatory signals, bacterial modification and the appearance of necrotising enterocolitis [5]. T lymphocytes adapt the premature intestinal mucosa to bacterial colonization to establish the inflammation. Enterocolitis displays an excessive intestinal mucosal lymphocytic reaction as the epithelial TLR4 signal to modify the pro-inflammatory T helper cell 17 and restrict the protective T regulatory cells. Formulation of the relevant platelet activating factor occurs in the impaired mucosa with a barrier malfunction. An intensified production and circulation of platelet activating factor with an inadequate platelet activating factor acetyl hydrolase, the enzyme which recruits the protein, is delineated [6].
Enteral Provisions
Parenteral nutrition modifies the pro-inflammatory genes. Intestinal bacterial flora and intestinal splanchnic
perfusion is interrelated with the parenteral nutrition. Parenteral prescriptions and human milk alter the
host genetic expression and methylation. The existence of intestinal flora, circulating endo-toxins, bacterial
blood culture/ sensitivity and 30% hydrogen content of the pneumatosis (gas exclusively produced by the
intestinal bacteria) implicate a bacteria induced pathogenesis [7]. Heterogeneous intestinal bacteria are
eradicated at the onset and the pathogenic bacteria predominate- facts noted in the premise of “dysbiosis”.
Interpretation
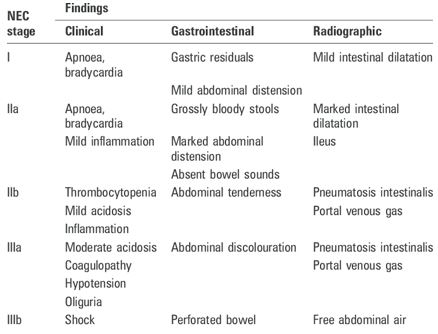
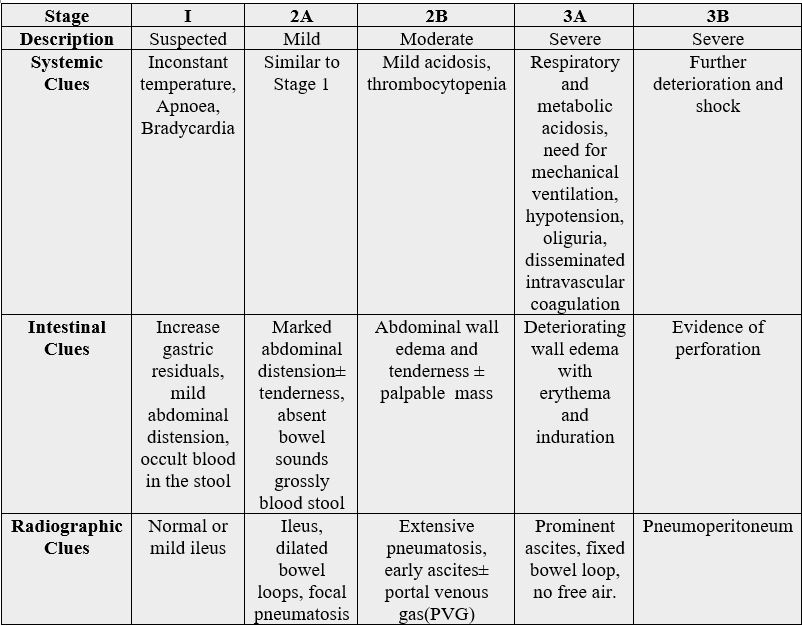
Interpretation is based on the precise clinical, radiographic and laboratory data. The classic presentation is a
robust preterm with a regular intake, abdominal distension, bloody diarrhoea and indications of sepsis.
Biomarkers
Biomarkers and non-invasive acute phase reactants such as the CRP (C Reactive protein) and proinflammatory
cytokines (Tumour necrosis factor TNF ᾳ, Interleukin 1 IL1 and Interleukin 6 IL6) are equivocal.
Organ specific biomarkers like the enterocyte impairment, intestinal barrier deterioration, intestinal fatty
acid binding protein, liver fatty acid binding protein, faecal calprotectin are requisite for early recognition of
the disease. The intestinal fatty acid binding protein (I-FABP) is a cytoplasmic protein with an enterocyte
lipid metabolite found in the circulation and secreted into urine following enterocyte injury. It is comparable
to the magnitude of intestinal necrosis and is a quantitative parameter. The I-FABP prevents the detection
of the inflammation as the plasma ½ life is fleeting and the normal range in preterm infants is variable.
Abundant necrosis with continuing injury has a decreased I-FABP because of a shortened half-life. Urinary
peptides and protein imply a poor prognosis [8]. Plain radiography is the elementary imaging technique
for diagnosis and ratification of necrotising enterocolitis. Abdominal ultrasound delineates pneumatosis
intestinalis and portal venous gas (PVG) earlier than a plain x-ray. Ultrasound demonstrates specific aspects
of perfusion, the gut wall diameter and motility and assists in predicting the disease progression and surgical
outcomes. The free intra-abdominal gas/ fluid and intestinal perforation is determined by the abdominal
ultrasound. The modality of colour doppler evaluates the celiac trunk and the blood velocity of the superior
mesenteric artery.
It delineates the poor perfusion and viability of the intestinal wall in potential patients [9]. Near infra-red spectroscopy (NIRS) demonstrates the progressive or inconclusive cases of intestinal inflammation. The skin probes of NIRS concentrate on the tissue to oxygenate the intestinal substratum instead of the entire length. Thus it assesses the splanchnic tissue oxygenation in the preterm infants with progressive intestinal inflammation and those lacking inflammation.
Preventive Policies
Breast milk diminishes the extent of enterocolitis. Human milk comprises of beneficial bioactive elements.
It restricts the TLR4 signal by prohibiting the glycogen synthase kinase 3β. The coordinated TLR4 signal
can thus activate of intestinal stem cell production and mucosal repair. The events are partly controlled by the
stimulation of epidermal growth factors signal receptors. Human milk components which safeguard against
the emergence of inflammation are i) Lactoferrin ii) Oligosaccharides and pre-biotics iii) Secretory IgA iv)
L-Arginine v) Nitrate/ Nitrite vi) Platelet activating factor acetyl hydrolase vii) Anti-oxidant factors viii)
Growth factors (Epidermal GF, Heparin binding EGF like GF, Transforming GF-β2 ix) Erythropoietin.
Donor breast milk acts as a replacement or additive to formula feeding is an efficient strategy for the preventing the condition.
Probiotics
Probiotics are live organisms that improve the bacterial milieu and the constitution of the host. The medium
prevents the development of inflammation, decreases the disease severity, extent and mortality in the
preterm [10]. Nevertheless, the specific agent, time of administration and duration of the therapy are have
not been specified. The pro-biotic bacteria lactobacillus ramonsus induces the enterocyte multiplication and
differentiation of the Paneth cells. The Cp G with the bacterial DNA triggers the Toll like receptor 9 and
inhibits TLR4. Thus a substitute to the live pro-biotic is available.
Therapeutics
Bell stage I or II inflammation is treated with pertinent supportive therapy i) termination of parenteral
nutrition ii) encourage ventilation iii) maintain fluid/ electrolyte and acid-base balance iv) adjust persisting
coagulopathy and/or thrombocytopenia v) bowel rest vi) optimal antibiotics for an appropriate duration [8].
Surgical Intervention
Surgical intervention is required in half the cases with inflammation and eliminates the necrotic intestine.
An abdominal drain and peritoneal irrigation is adequate in some. Laprotomy with peritoneal drainage
produces similar results. Primary peritoneal drainage is restricted to patients with elevated intra-abdominal
pressure or for very small infants (< 750gm).
Current Modalities
Pentoxifylline with antibiotics is recommended for treating neonatal sepsis and prospective necrotising
enterocolitis 0.62. Intra-peritoneal pentoxifylline decreases the extent and severity of the infection.
Consequences
The mortality of necrotising entero-colitis is 20%-30%. Infants with a surgical intervention delineate a 50
% mortality regardless of the proficient surgical and medical. The intestinal or systemic damage presents
survivors with co-morbidities [8].
Complications
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!