Biography
Interests
Nighat Sultana*1, Muhammad Saleem Qazi1 & Alay Khan2
1Pharmaceutical Research Center, Pakistan Council of Scientific and Industrial Research (PCSIR) Laboratories Complex, Karachi-75280, Pakistan
2Crop Diseases Research Institute, PARC, University of Karachi, Karachi-75270 - Pakistan
*Correspondence to: Dr. Nighat Sultana, Pharmaceutical Research Center, Pakistan Council of Scientific and Industrial Research (PCSIR) Laboratories Complex, Karachi-75280, Pakistan.
Copyright © 2018 Dr. Nighat Sultana, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The nematicidal effect of ethyl alcohol extract of Camellia sinensis (Green tea) leaves were tested against Helicotylenchus indicus, Xiphinema americanum and Xiphinema index at the concentrations of 1.0 and 0.1%, extract was used in distill water and observation taken at different time periods and compared with chemical neamticide carbofuran. There were four replicates of each test conducted by taking conc. percentage 1.0 and 0.1 and mortality was recorded. Nematicidal mortality was 100% with carbofuran chemical nematicide at different time periods at same time ethanol extract also shown nematicidal property at different time periods on above nematodes and with the use of 1% concentration of Camellia sinensis leaves ethyl alcohol extract applied on Helicotylenchus indicus, after 1 hour and 24hours and observed 50 mortality in 1hour and 22.5 mortalities during 24hours and 0.1% concentration of extract application 2.5 for 1hour and 6.25 for 24hours. Same nematicidal activity test conducted on Xiphinema americanum and Xiphinema index and recorded mortality level at 1% extract concentration 50 and 46.25, where 0.1 extract concentration shown 0.0 and 6.25 mortalites in 1hour, 24hour extract application of 1% and 0.1% are as 63.25, 0.0 and 20.0, 0.0. The plant is economic importance having nematicidal value. These results showed that the extract obtained from Camellia sinensis have potent nematicidal activity and may be responsible for the antinematicidal activity of whole plant.
Introduction
Camellia sinensis L. commonly called tea is largely consumed since time of immemorial and has immense
scientific significance for its numerous therapeutic properties mainly because of polyphenolic compounds
[1]. Only young leaves are mostly used for drinks and extracts, however many parts of this plant are being
explored for benefit of mankind [2]. Depending on tea manufacturing methods, tea is divided mainly into:
green and the black one [3]. Green tea is generally safe, non-toxic and having no side effects after use [4].
Attempt of botanical insecticides have been in practice since long in some form or the other for controlling
insect pests [5]. The current study is undertaken to evaluate the potential of various crude Camellia sinensis
(green tea) extracts as nematicidal agent.
Nematodes are the most numerous multi-cellular animals on earth. Nematodes are structurally simple organisms. Adult nematodes are comprised of approximately 1,000 somatic cells, and potentially hundreds of cells associated with the reproductive system. Nematodes have been characterized as a tube within a tube, referring to the alimentary canal which extends from the mouth on the anterior end, to the anus located near the tail. Nematodes possess digestive, nervous, excretory, and reproductive systems but lack a discrete circulatory or respiratory system. In size they range from 0.3mm to over 8 meters.
Plant-parasitic nematodes constitute one of the most important pest groups of the economic crops, especially in the developed and developing countries of the world. The use of plants and plant products is one of the promising methods for nematode control. They are cheap, easy to apply, produce no pollution hazards and have the capacity to structurally and nutritionally improve the soil health. In view of these facts, investigations have been undertaken by various groups of scientists (Gommers, 1981; Qamar et al., 1995; Nogueira et al., 1996) which showed an effective control of rootknot nematodes. In the present article, studies on nematicidal activity of alcoholic extract from the air-dried aerial parts of Camellia sinensis L. is described.
Helicotylenchus indicusis a genus of nematodes in the family Hoplolaimidae [6]. They are known generally as spiral nematodes [7]. They are found worldwide [8]. They are among the most common parasitic nematodes of plants [7]. Most are ectoparasites of plant roots. They insert their stylets into root epidermis to feed. Some species live half-buried in the root tissue, and others penetrate the root and live inside. They lay eggs on, around, or inside the roots, and within two or three days the juveniles emerge to feed [7]. The genus is found on a wide variety of host plant taxa [8]. The genus is found on a wide variety of host plant taxa [8].
Most species are not very damaging to the plant. Nematodes of this genus have been noted to be ubiquitous in soil samples in Florida without plant damage nearby. Four species out of over 200 are known as destructive plant pests that suppress plant growth: H. dihystera, H. multicinctus, H. pseudorobustus and H. digonicus. A few others are potential pests [7].
Plants infested with aggressive species may become stunted and yellowed, but usually there is no sign of infestation in the herbage. An exception is in parasitism by H. multicinctus, which can cause enough root necrosis that it seriously weakens the plant. This species may be the most economically important occurring in crops such as bananas of the Cavendish group. Other species have caused occasional damage to maize and Kentucky blue grass [7].
Xiphinema americanum (American dagger nematode) is a plant pathogenic nematode. It is one of many species that belongs to the genus Xiphinema. It was first described by N. A. Cobb in 1913, who found it on both sides of the United States on the roots of grass, corn, and citrus trees [9]. Not only is Xiphinema americanum known to vector plant viruses, but also X. americanum has been referred to as “the most destructive plant parasitic nematode in America”, and one of the four major nematode pests in the Southeastern United States [10,11,12].
This species of nematode is also found to be sensitive to soil pH and they are found most frequently in soils with a pH of 6.0 or higher [13]. Control of the American Dagger Nematode presents problems because X.americanum is hard to completely remove. Nematicides generally remove up to 95% of the nematodes in soil, however the 5% that remain can reproduce asexually and the viruses that they carry can still infect the roots of young plants. Therefore, to eliminate the nematodes, nematicides should be used along with having a bare soil field for at least 2-years period. This ensures that the X. americanum has no food source. At the end of this 2-year period the nematodes should be eradicated [14].
The spraying of nematicides also causes plants to release allelopathic chemicals [15]. These chemicals then kill the nematodes by active suppression because they are toxic to the nematode. Crop rotation is another form of control for X. americanum. It has been shown that certain non-host plants may deny the nematode population an adequate food source for reproduction, and thus greatly reduce its population in the soil. This is termed passive suppression [16]. X. americanum can only travel via run-off and in damp soil, therefore if soils are kept dry enough the nematodes can be localized [15]. There is also evidence of X. americanum resistance and “tolerance” seen in certain species of grapes that appeared to be better adapted to the parasite [17].
Xiphinema index (California dagger nematode) is a plant-parasitic nematode. A major pest of grapes, the California dagger nematode provided the first example of a nematode acting as a vector for a viral plant disease. It has spread to multiple continents where there is viticulture production. Xiphinema index is a migratory ectoparasite that primarily feeds on the root tips of grapes (Vitis vinifera). The body of a female is around 3mm long, and the odontostyle is approximately 126um long. There is a thick cuticle with thin striations across the body. The female has one or two ovaries that are typically paired. Males and females both have dorsally rounded tails that are short [18]. Xiphinema index is a migratory ectoparasite that primarily feeds on the root tips of grapes (Vitis vinifera).
The body of a female is around 3mm long, and the odontostyle is approximately 126um long. There is a thick cuticle with thin striations across the body. The female has one or two ovaries that are typically paired. Males and females both have dorsally rounded tails that are short [7]. Feeding on a susceptible host causes the root stunting and tip galling. Furthermore, it is a vector of the grapevine fan leaf virus. Other hosts of this parasite include fig, apple, rose, pistachio, as well as several others. Infected roots should be removed from the vineyard, and a non-host should be grown for several years, if possible. Historically, this pest has been managed by the use of chemical fumigants, such as 1,3-Dichloropropene and Methyl Bromide. There are few post-plant chemical options to manage this pest. These fumigants must be applied deeply in the soil to be effective, as the nematode can reside deeply in the soil and thus resist attempts to eradicate it completely. Root stocks that are bred for resistance to both GFLV and X. index have proven to be effective at managing the disease. An effective rootstock must be resistant to both the virus and the nematode. Hot water treatments of root stocks to be planted ensures there will be little chance of the introducing the disease into the field. Preventing the introduction of infected plant or soil material is essential to manage both X. index and GFLV [18]. Camellia sinensis L. commonly called green tea is largely consumed since time of immemorial and has immense scientific significance for its numerous properties mainly because of polyphenolic compounds [19]. Only young leaves are mostly used for drinks and extracts, however, many parts of this plant are being explored beneficial [20]. Green tea is generally safe, non-toxic and having no side effects after use [21]. Attempt of botanical insecticides have been in practice since long in some form or the other for controlling insect pests [22]. The current study is undertaken for to evaluate the potential of various crude Camellia sinensis (green tea) extracts as Nematicidal activity.
Result and Discussion
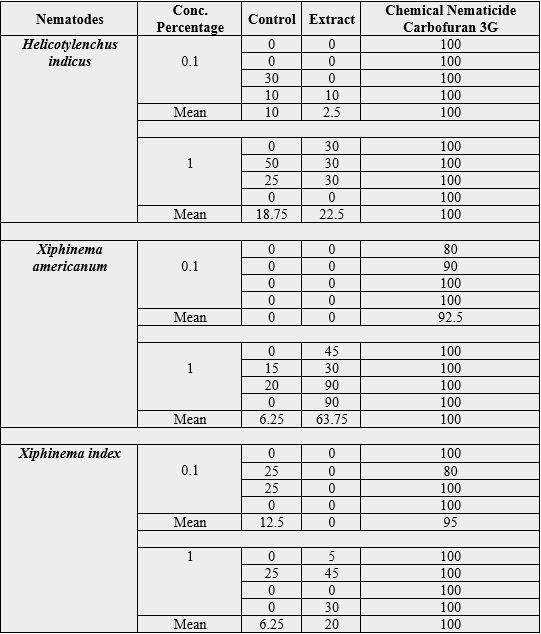
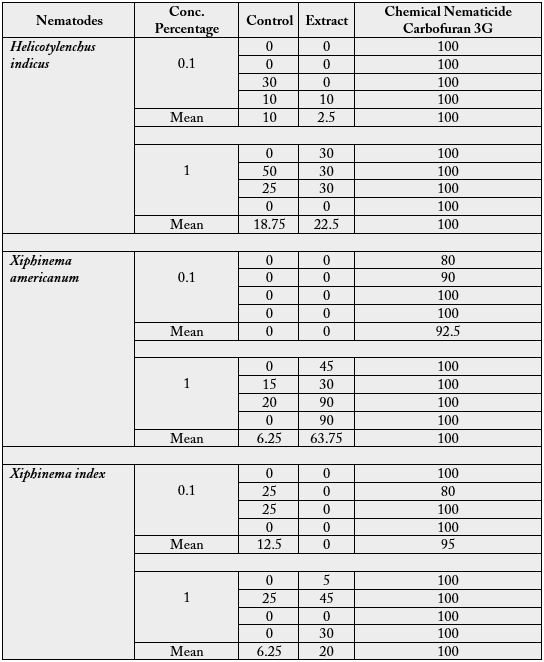
The nematicidal activity of ethanol fractions of leaves extract of Camellia sinensis 1 and 24 hours were tested
against Helicotylenchus indicus, Xiphinema americanum and Xiphinema index. The total alcohol soluble crud extract Camellia sinensis leaves, were screened for its nematicidal activity against nematodes Helicotylenchus indicus, Xiphinema americanum and Xiphinema index. And results were obtained for the nematicidal activity of ethanol extract.
The crude ethanol Camellia sinensis (Green Tea) showed 6.25% mortality at 0.1% concentration after 1hr, while 2.5% mortality at 0.1% concentration after 24hrs whereas, 1% ethanol soluble extract showed 50% mortality after 1hr and 22.5% mortality after 24hrs, and carbofuran chemical nematicide shown 100% at both concentration and time periods while simply controlled showed 3.75% mortality at 0.1% concentration and 11.25% at 1.0% concentration at 1hr, where 0.1% concentration shown 10% mortality and 1.0% showed 18.75% after 24 hrs. When extract were applied on the Xiphinema americanum which showed 0% mortality at 0.1% concentration after 1hr, while 0% mortality at 0.1% concentration after 24hrs, whereas, 1% ethanol soluble extract showed 56.25% mortality after 1hr and 63.75% mortality after 24hrs, where carbofuran chemical nematicide shown 90% at 0.1% concentration and 100% mortality at 1.0% after 1hr where 92.5% at 0.1% concentration and 100% at 1.0% concentration after 24hrs while controlled showed 10% mortality at 0.1% concentration and 15% at 1.0% concentration after 1hr, where 0.1% concentration shown 0% mortality and 1.0% showed 6.25% after 24 hrs
Application of ethanol extract on Xiphinema index showed 0% mortality at 6.25% concentration after 1hr, while 0% mortality at 0.1% concentration after 24hrs, whereas, 1% ethanol soluble extract showed 46.25% mortality after 1hr and 20% mortality after 24hrs, while carbofuran chemical nematicide shown 92.5% at 0.1% concentration and 97.5% mortality at 1.0% after 1hr, where 95% at 0.1% concentration and 100% at 1.0% concentration after 24hrs, while controlled showed 5% mortality at 0.1% concentration and 1.75% at 1.0% concentration after 1hr, where 21.5% concentration shown 0% mortality and 1.0% showed 6.25% after 24 hrs.
According to collected data higher nematicidal activity was observed on Xiphinema americanum 63.75 at 1.0% concentration after 24hrs and 56.5% at 1% concentration after 1hr, where 0% at 0.1% concentration after both time periods was observed. While moderate result obtained by applying ethanol extract on Helicotylenchus indicus which is 50% at 1.0% conc. and 6.25 at 0.1% conc. after 1hr but got less mortality while applied for 24hrs, which shown 2.5% at 0.1% conc. and 22.5% at 1.0% conc. Where mortality in Xiphinema index showed 6.25% at 0.1% conc. and 46.25% at 1.0% conc. after 1hr, 0% at 0.1% conc. and 20% at 1.0 conc. after 24hrs.
The nematode population was most abated for all the three nematodes namely Helicotytenchus indicus, Xiphinema index and Xiphinema americunum in 1% concentration of extract. The efficacy of extract at 1% was in the order H. indicus < X. index < X. americanum. At 0.1% concentration no control was observed of X. americanum while X. index population was reduced to a higher degree as compared to that of H. indicus. Detailed results of replicate application with different concentrations for different time periods and comparisons with chemical nematicide are given in table1 and 2.
a) Mortality (%) in different treatments against three nematodes.
b) Four replicates were used for each treatment.
a) Mortality (%) in different treatments against three nematodes.
b) Four replicates were used for each treatment.
Experimental
Nematodes were isolated from a Grapevine orchard of Kalat, Balochistan province, Pakistan. The nematodes
were isolated using a modified (Cobb, 1918) [16] decanting and selective sieving method at Crop
Diseases Research Institute, PARC, University of Karachi.
0.1% and 1% concentration of extract was used in distill water and observation taken at different time periods (Table-1,2). There were four replicates. The mortality was recorded under a stereoscopic microscope in a glass counting chamber.
Helicotylenchus indicus occurring in crops such as bananas of the Cavendish group. Other species have caused occasional damage to maize and Kentucky bluegrass [10]. Xiphinema americanum The most common plant hosts infected by X. americanum are common weeds and grasses, strawberries, soybeans, forest trees (spruce, pine, etc.), perennial orchards, and grapes. This broad host range is due to the genetic diversity within the X. americanum species.
This species of nematode is also found to be sensitive to soil pH, and they are found most frequently in soils with a pH of 6.0 or higher [23]. where X. americanum is found include Australia, Belize, Brazil, Chile, Guatemala, India, Japan, Korea Democratic People’s Republic, Korea Republic, Mexico, New Zealand, Pakistan, Panama, South Africa, Sri Lanka, Uruguay, and areas of the Caribbean as well [24]. Xiphinema index Furthermore, it is a vector of the grapevine fan leaf virus. Other hosts of this parasite include fig, apple, rose, pistachio, as well as several others [19].
Air-dried aerial parts of Camellia sinensis (15kg dry weight) were dried and extracted with EtOH (100L).
The EtOH extract was concentrated to a gum (813g), dissolved in distilled water.
Experiments were performed under laboratory conditions at 28 + 2°C. Fresh egg masses collected from stock culture maintained on tomato root tissue were kept in water for egg hatching. The larvae emerged after 48h from egg masses incubated at 30°C and were used as test species for larval mortality studies.
The movements of nematodes were checked by touching them with needle. For the nematicidal activity of leave extract, stock solutions (30mg/ml) of Camellia sinensis (Green tea) in ethyl alcohol (AL-AS.) were prepared. To determine nematicidal effect of extract, 100 freshly hatched second stage juveniles were taken in 5ml tap water.
Measured amount of stock solution were added to make dilution of 1 and 0.5%. Standard nematicide Carbofuran 1 and 0.5% were taken for comparision and tap water taken as control. After 1 and 24 h exposure with Camellia sinensis fraction, the larvae were counted for mortality and non-mortality under stereoscopic microscope. The deaths of nematodes were confirmed by keeping them in tap water for 24h. The percent mortality was worked out from an average of three replicates. The result of percent mortality in different fraction of Camellia sinensis after 1 and 24h of leaf extract were given in Table 1 and 2.
Conclusion
The plant is of economical importance and has nematicidal value. Phytochemicals are used in many medicines,
insecticides, pesticides, especially for plant diseases. Those plants have these proportions can be used
in the manufacture of nematicide (Javed et al., 2006). It is evident from the above discussion that there
is a great likelihood of use of bio-control agents for disease control by nematodes (Javed,et al., 2005).
Although, several potential bio-control agents have been isolated and tested for their efficacy against soil
born root pathogens, there is need to discover new potential antagonists or improve strains of already
isolated antagonists for better crop production (Jiskani et al., 2005). Development of a sample, cheap and
effective method for mass production of bio-control agents is a pre-requisite for the replacement of chemical
fungicides by a bio-control agent which also needs investigation [25].
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!