Biography
Interests
Dr. Shimon Shatzmiller*, Dr. Inbal Lapidot & Dr. Galina Zats
Department of Chemical Sciences, Ariel University, Ariel Israel
*Correspondence to: Dr. Shimon Shatzmiller, Emeritus Professor, Department of Chemical Sciences, Ariel University, Ariel Israel.
Copyright © 2019 Dr. Shimon Shatzmiller, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Neurodegenerative diseases for example type 2 diabetes mellitus (T2DM), obesity, Parkinson’s disease (PD) and Alzheimer’s a disease (AD) represent the biggest global health challenges of this century and are associated with significant comorbidities and healthcare costs [1]. Although multiple factors undoubtedly contribute to the development and progression of DM and obesity, research over the last decade has demonstrated that the microbes that colonize the human gut may play key contributory roles. Gut microbes are now known to co-develop with the human host and are strongly influenced by mode of birth and early diet and nutrition, as well as environmental and other factors including antibiotic exposure [2]. Gut microbes contribute to human health through roles in polysaccharide breakdown, nutrient absorption, inflammatory responses, gut permeability, and bile acid modification. Numerous studies have suggested that disruptions in the relative proportions of gut microbial populations may contribute to weight gain and insulin resistance, including alterations in Gammaproteobacteria and Verrucomicrobia and the ratios of Firmicutes to Bacteroidetes in weight gain and possible alterations in butyrate-producing bacteria such as Faecalibacterium, prausnitzii in DM. Also, it has been shown that the methanogenic Archaea may contribute to altered metabolism and weight gain in the host. However, the majority of studies are performed with stool or colonic samples and may not be representative of the metabolically active small intestine. Studies predominantly in rodent models are beginning to elucidate the mechanisms by which gut microbes contribute to DM and obesity, but much remains to be learned before we can begin to approach targeted treatments.

Foreword
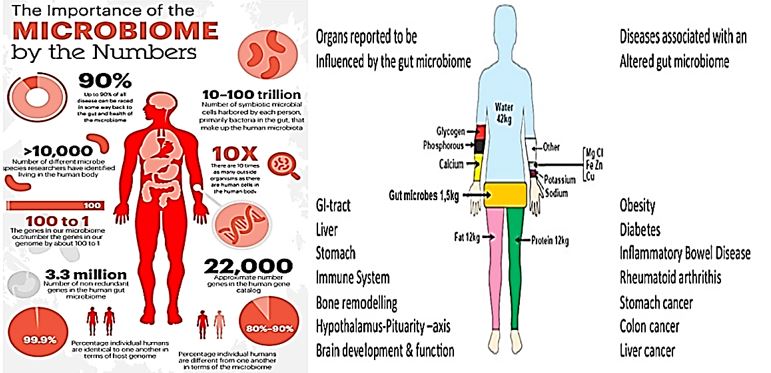
The human microbiome is the collection of microbes that colonize the human body.
However, the human organism is a living example of many organisms that survive a symbiosis in equilibrium. There are two main sorts of organisms in that partnership: the human cells and the microbiota.
The Balance of Numbers is on the Side of the Partners, the Microbes:
Of the trillions of cells taking partnership, only 30% are human cells. Right now, your body is teeming with
microscopic organisms. In fact, they outnumber our cells by ten to one. Thousands of different microbial
species colonize our bodies and together contain over one hundred times more genes than in our genome.
This group of microorganisms is called the microbiome and helps keep us alive.
Studying microbes within their communities rather than individually play a key role in understanding the human microbiome. A major goal of the Human Microbiome.
If the balance between the symbiotic partner retaines, the combined organism can continue with their regular life. When this is disturbed either by the changes in numbers of cells or location in the body, dysfunction occurs. And the combined organism may become a parish. An Ending of a beautiful partnership.
The Gut Microbiome and Health
Tens of trillions of microorganisms live in our gut, comprising at least 1,000 species of known bacteria and
more than 3 million genes. While one-third of gut microbes are common to most individuals, the remaining
two-thirds are specific to each of us; they evolve throughout a lifetime, dependent on diet and other lifestyle
and environmental exposures.
Increasingly, researchers are learning how changes to gut microbiome impact overall health and the risk of disease; studies have uncovered links between altered gut microbiota and obesity and cancer, as well as conditions that affect the brain, including autism, anxiety, and depression.
Many research teams have strengthened the gut-brain link with their new research, after revealing how the gut microbiome may be involved in Parkinson’s disease.
Previous studies have shown that the gut microbiome is altered in patients with Parkinson’s disease, and these individuals often experience constipation and other gastrointestinal (GI) problems years before the onset of motor problems.
“Remarkably, 70 percent of all neurons in the peripheral nervous system - that is, not the brain or spinal cord - are in the intestines, and the gut’s nervous system is directly connected to the central nervous system through the vagus nerve,” notes Mazmanian.
“Because GI problems often precede the motor symptoms by many years, and because environmental factors cause most PD [Parkinson’s disease] cases, we hypothesized that bacteria in the gut might contribute to PD.”
Introduction
Recently, the scientific community has come to embrace the important role that bacteria have in fostering
a strong immune system and keeping us healthy [3]. Not only are all bacteria not detrimental to our health,
but some are crucial for boosting immunity, keeping our digestive systems running smoothly, our hormone
levels balanced and our brains working properly.
More research is needed that involves the microbiome of the gut, pulmonary systems, the skin for the example, with regards to how these microbiomes can influence neurological health either directly or indirectly. Humans contain a complex and dynamic community of microbes called the microbiome that forms a “meta-organism” with symbiotic or commensal benefit to the host.
Recent interest in the role of the microbiome in human health and disease has rapidly expanded over the last several years with the advent of new sequencing and bioinformatics technologies for interrogating the genetics of complex microbial communities and microbial-host interactions. There is currently much interest in the ability of Gastro-Intestinal (GI) tract bacteria to influence host innate-immune, neuroinflammatory, neuro-modulatory and neurotransmission-functions.
Here we list ten recent, highly specific and illustrative insights into the potential contribution of pathogenic microbes, altered microbiome signaling and other disease-inducing agents to the development of Alzheimer’s Disease (AD):
1. Fungal infection of the CNS
2. HSV-1 is associated with the AD
3. Prion diseases (4) Chlamydophila pneumonia, other pathogenic bacteria and AD
4. Chlamydophila pneumoniae, other pathogenic bacteria, and AD
5. HIV-1 and AD.
6. Toxoplasma and neurodegeneration.
7. Viroids, miRNAs, and AD.
8. Hepatitis and AD.
9. Cytomegalovirus and AD.
10. GI tract and blood-brain barrier permeability.
What Is the Microbiome?
The microbiome refers to the collection of microorganisms that colonize the human body and includes
bacteria, viruses, and fungi. These microorganisms tend to inhabit specific sites in the body called physiological
niches, where conditions are suitable for growth.
For instance, Staphylococcus aureus is typically found on the skin due to the slightly acidic pH of the skin and the abundance of oxygen. In the human gut, where conditions are often anaerobic, bacteria such as Lactobacilli are found.
Human beings have clusters of bacteria in different parts of the body, such as in the surface or deep layers of skin (skin microbiota), the mouth (oral microbiota), the vagina (vaginal microbiota), and so on. Each of us has a complex internal ecosystem of bacteria located within our bodies that we call the microbiome. The microbiome is defined as-as “community of microbes. Although Gut microbiota (formerly called gut flora), is the name given today to the microbe population living in our intestine. Our gut microbiota contains tens of trillions of microorganisms, including at least 1000 different species of known bacteria with more than 3 million genes (150 times more than human genes). Microbiota can, in total, weigh up to 2kg.
One-third of our gut microbiota is common to most people, while two-thirds are specific to each one of us. In other words, the microbiota in your intestine is like an individual identity card.
The human microbiota consists of the 10–100 trillion symbiotic microbial cells harbored by each person, primarily bacteria in the gut. The human ‘microbiome’ consists of the genes these cells harbor we live in symbiosis with this microbiome. Some differentiation is to be found between the many sorts of microbiota like the skin Microbiome and the Gut Microbiome, for example.
Gut microbes contribute to human health through roles in polysaccharide breakdown, nutrient absorption, inflammatory responses, gut permeability, and bile acid modification. Numerous studies have suggested that disruptions in the relative proportions of gut microbial populations may contribute to weight gain and insulin resistance, including

Alterations in Gammaproteobacteria and Verrucomicrobia and the ratios of Firmicutes to Bacteroidetes in weight gain and possible alterations in butyrate-producing bacteria such as Faecalibacterium, prausnitzii in DM. Also, it was shown that the methanogenic Archaea might contribute to altered metabolism and weight gain in the host. However, most of the studies are performed with stool or colonic samples and may not be representative of the metabolically active small intestine. Studies predominantly in rodent models are beginning to elucidate the mechanisms by which gut microbes contribute to DM and obesity, but much remains to be learned before we can begin to approach targeted treatments [4a,4b].
The skin is the human body’s largest organ, colonized by a diverse milieu of microorganisms, most of which are harmless or even beneficial to their host. Colonization is driven by the ecology of the skin surface, which is highly variable depending on topographical location, endogenous host factors and exogenous environmental factors. The cutaneous innate and adaptive immune responses can modulate the skin microbiota, but the microbiota also functions in educating the immune system. The development of molecular methods to identify microorganisms has led to an emerging view of the resident skin bacteria as highly diverse and variable [5].
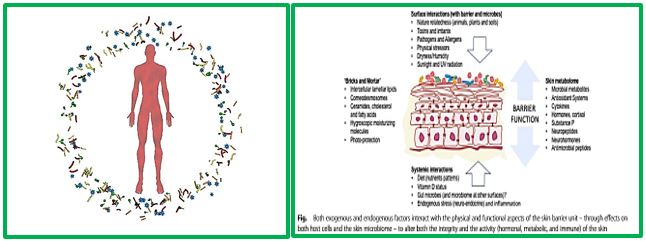
The microbiome is the collective term used to refer to all of the microbes found in or on the human body and more specifically, the genomes of those.
The microorganisms that colonize the human body have traditionally been thought of by doctors as requiring elimination. Viruses have been associated with colds and flu and bacteria with infections such as strep throat.
However, most microbes do not cause ill health, and the more researchers learn about them, the more is understood about how important the balance of different microbial populations is within the context of health and disease. Imbalance of the microbial populations can lead to health problems and correct that imbalance may resolve such problems.
This improved understanding may lead to the development of more effective treatment approaches. Some examples of the conditions that are known to involve the microbiome are described in more detail below:
Acne, Diarrhea Associated with Antibiotic Use, Allergies, Autoimmune Disease, Cancer, Diabetes
Some researchers theorize that the increase in diabetes prevalence may be connected to an increase in the
amount of sugar and fat in the diet increases, compared to a few decades ago, since these dietary changes
could alter which types of microbes can survive in the gut.
We know that in obese people the bacterial population in the gut is different, and the different population of bacteria may lead to a vicious cycle contributing to obesity. Researchers had shown that when obese men with insulin resistance were given microbes from men who were thin and healthy, the obese men became more responsive to insulin, which resulted in healthier blood glucose levels [6]. Focusing on the human gut, the microbiome in health is useful for several functions, including digestion of food, protecting the intestinal barrier and engaging the local immune response. This results in a metabolic factory that can communicate with the organs of the body. Bacteria in the gut can influence organs that are near, such as the liver and the bowels but also organs that are far away, such as the brain [7].
Autoimmune Diseases
Autoimmune diseases are conditions that arise because the immune system launches an attack against its
own cells and therefore non-foreign bodily tissues. In the case of multiple sclerosis, the myelin sheath that
surrounds and protects nerves is attacked, and in rheumatoid arthritis, connective tissue in the joints is
targeted.
As is the case with allergy, this is likely to arise when the immune system does not learn to differentiate between damaging substances and harmless ones. Some types of gut microbes may protect against autoimmune diseases, while others appear to increase susceptibility to the conditions. In murine models of type 1 diabetes, where cells in the pancreas are attacked, certain microbes have been shown to protect against the disease.
Most of Parkinson’s disease (PD) patients suffer from gastrointestinal symptoms of which constipation is considered the most prominent [8]. Recently, in addition to constipation, a diagnosis of irritable bowel syndrome (IBS) was also found to be associated with increased PD risk. Gut microbiota alterations have been reported in IBS and recently also in PD. IBS-like bowel symptoms in PD and their possible connection to other nonmotor symptoms and fecal microbiota were assessed.
Scientists have discovered for the first time a functional link between bacteria in the intestines and Parkinson’s disease (PD) [9a,9b]. The researchers show that changes in the composition of gut bacterial populations–or possibly gut bacteria themselves-are actively contributing to and may even cause the deterioration of motor skills that is the hallmark of this disease.
By the time a person with Parkinson’s notices something is wrong, the microbes in his gut may have long known about it. Reporting April 28 in Genome Medicine, researchers led by Ullrich Wüllner of the University of Bonn in Germany describe striking changes in the microbial communities living in the intestines of people in the earliest stages of the disease. Based on specific shifts of microbial species in the PD gut, the researchers speculated that the microbiome could interfere with the production of short chain fatty acids, or the permeability of the mucosal gut barrier-two functions intimately linked with inflammation and potentially with neurodegeneration.
To test if bacteria in the gut may contribute to PD, the researchers utilized mice that overproduce αSyn and display symptoms of Parkinson’s. One group of mice had a complex consortium of gut bacteria; the others, called germ-free mice, were bred in a completely sterile environment and thus lacked gut bacteria. The researchers had both groups of mice perform several tasks to measure their motor skills, such as running on treadmills, crossing a beam, and descending from a pole. The germ-free mice performed significantly better than the mice with a complete microbiome.
When gut bacteria break down dietary fiber, they produce molecules called short-chain fatty acids (SCFAs), such as acetate and butyrate. Previous research has shown that these molecules also can activate immune responses in the brain. Thus, the researchers hypothesized that an imbalance in the levels of SCFAs regulates brain inflammation and other symptoms of PD. Indeed, when germ-free mice were fed SCFAs, cells called microglia–which are immune cells residing in the brain–became activated. Such inflammatory processes can cause neurons to malfunction or even die. In fact, germ-free mice fed SCFAs now showed motor disabilities and αSyn aggregation in regions of the brain linked to PD.
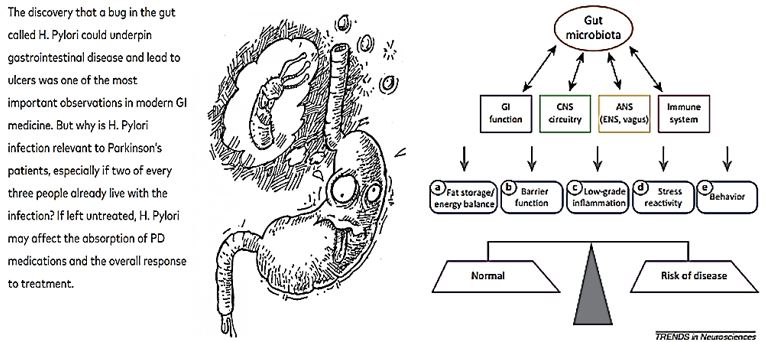
Bidirectional communication between gut microbiota and components of the gut–brain axis influence normal homeostasis and may contribute to risk of disease [10].
Below Are Key Points About the Relationship Between Gut Bacteria and Parkinson’s:
1. It is still unknown if H. Pylori or other gut bacteria are a risk factor for the later development of PD.
2. If a person with Parkinson’s is experiencing motor fluctuations that cannot be controlled by medication
adjustment, ask your doctor (or GI specialist) for a C-urea breath test to test for H. Pylori infection.
3. Because H. Pylori is such a common infection (occurring in two out of every three people), the decision
to treat it should be made in consultation with a GI specialist and neurologist. A GI specialist may need to
perform follow-up tests.
4. Changes in your Parkinson’s symptoms, motor fluctuations and medications should all be managed by
your neurologist before and after H. Pylori treatment.
5. The new findings on Prevotellaceae and Enterobacteriacea will need further investigation and have not
shown to interfere with current PD treatment.
6. The human microbiome may be a risk factor for PD, but until clinical studies are conducted, we do not
recommend that people with PD seek treatment with antibiotics, which could advance drug resistance and
other issues.
We do not know what causes changes in the microbiome in Parkinson’s, theorized factors include: intestinal absorption, problems with gastric motility or diet. The exact causes remain unknown.
Roles of Gut Microbes in the Development of Obesity
Disruptions in the normal balance of gut microbial populations, termed dysbiosis, has been linked to a
variety of gut-related diseases and conditions, including inflammatory bowel disease, and nonalcoholic fatty
liver disease, as well as DM and obesity [11a-13b]. In the first studies, linking gut microbes to weight gain
were performed in mouse models, and showed that conventionally raised animals had 42% more total body
fat than germ-free (GF) mice, even though the GF mice consumed 29% more chow.40 “Conventionalizing”
the GF mice (by spreading cecal contents from conventionally raised donors on their fur and allowing them
to lick it off) resulted in a 57% increase in total body fat content and a 61% increase in epididymal fat weight
in the recipients,40 despite decreased chow consumption[14a-14d]. Using cecal contents from genetically
obese (ob/ob) mice resulted in an even greater increase in body fat.35 These data underscored that different
gut microbial populations could have different effects on host weight gain and suggested the potential for
the development of therapeutic strategies based on targeting (or augmenting) specific microbial populations
Subsequent studies focused on identifying gut microbial populations that were altered in obesity. Firmicutes
and Bacteroidetes are the dominant gut phyla in both mice and humans, and while there are conflicting
data, several studies have demonstrated an association between lower levels of Bacteroidetes (or lower
Bacteroidetes to Firmicutes ratios) and obesity [15a,15b]. Obese human subjects were shown to have lower
levels of Bacteroidetes than lean subjects, which increased following weight loss on both low-fat and lowcarb
diets in a manner that correlated with a percentage loss of body weight rather than changes in dietary
calorie content over time [16]. Dieting and Roux-en-Y gastric bypass (RYGB) have been shown to result
in decreases in Firmicutes (genera Lactobacillus and Clostridium) and increases in Bacteroidetes (genera
Bacteroides and Prevotella), as well as increases in Gammaproteobacteria (Escherichia) and Verrucomicrobia
(Akkermansia) in human subjects [17]. Taken together, these studies suggest that changes in specific gut
microbial populations can directly affect host weight gain [18a-18c].
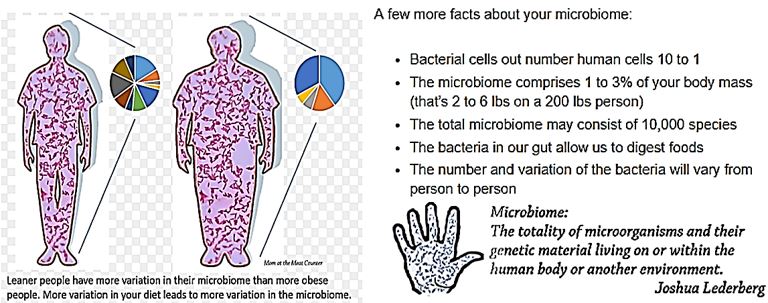
How Do We Change Our Microbiome?
The microbiome will change based on what you feed it [19]. If you eat a diet of fat and sugars, your microbiome
will adjust to digesting fat and sugar. If you eat more leafy greens and vegetables, the bacteria will change to
digest those. So, the more variety in your diet, the more variety in your microbiome.
Medicines like antibiotics can also change your microbiome. Everyone has been a little sick to their stomach after taking antibiotics (or you’ve had a sick child that has developed a nasty diaper rash after being on antibiotics). Even the bacteria on your skin can be affected by antibiotics. This is why doctors suggest that you eat yogurt after you’ve had to take antibiotics. Yogurt is full of healthy bacteria, and you need to repopulate your gut with healthy bacteria after you’ve had antibiotics.
Scientists are figuring out new ways to alter the microbiome in humans and animals all the time. Foods and supplements that can encourage the growth of good bacteria and discourage the growth of bad bacteria are available. Of course, we all know the benefits of eating yogurt and drinking acidophilus milk (yogurt). Cattle farmers have been using feed additives called ionophores to optimize the microbiome in their stomachs and help cattle digest feed more efficiently for years.
Dr. Power said that pretty soon, human health efforts will encompass care for the human as well as care for the microorganisms that live on and within the human. We may be able to treat chronic diseases in humans by treating and altering the bacteria that live within them [20].
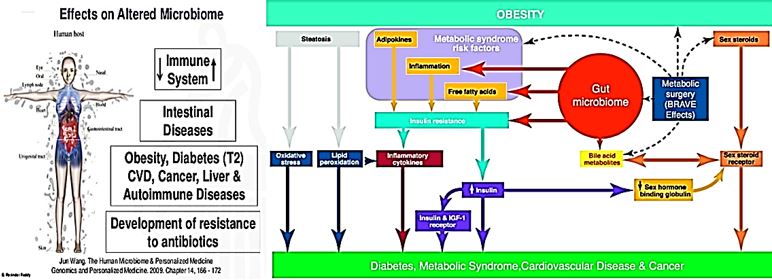
Roles of Gut Microbes in the Development of Diabetes Mellitus
In addition to the above-described potential roles of gut microbes in the development of obesity, alterations
in gut microbial populations have been linked to changes in insulin sensitivity, altered glucose metabolism,
and the development of the metabolic syndrome and T2DM [21]. The first such study, performed by Larsen
et al. in a Danish cohort, identified decreased overall microbial diversity in subjects with T2DM, decreases
in Firmicutes (including Clostridia), and enrichment in Proteobacteria that correlated with plasma glucose
on oral glucose tolerance test. Interestingly, they identified a negative correlation between Bacteroidetes to
Firmicutes ratios and BMI but a positive correlation between Bacteroidetes to Firmicutes ratios and plasma
glucose, which the authors suggested might indicate that obesity and DM are associated with different
microbial populations [22]. Subsequent metagenomic studies by Qin et al. in a Chinese cohort and by Karlsson
et al. in a cohort of 70-year old European women89 identified some comparable changes in gut microbes, as
well as metagenomic markers that could accurately discriminate between subjects with T2DM and controls
[23]. Karlsson et al. identified decreases in Clostridium species, similar to Larsen et al., as well as increases
in Lactobacillus species, and demonstrated positive correlations between Clostridium species and fasting
glucose and negative correlations for Lactobacillus species. Qin et al. also observed decreases in butyrateproducing
bacteria, such as Faecalibacterium prausnitzii, Roseburia intestinalis, Roseburia inulinivorans,
and Eubacterium rectale increases in bacterial genes related to oxidative stress in diabetic subjects [24].
That there were also some differences in metagenomics markers between the 2 populations is unsurprising
given the disparity in age and ethnicity between the cohorts, further underscoring the influences of age,
environment, and diet on the microbiome. A later study by the same Chinese group compared microbial
populations in individuals with normal glucose tolerance, prediabetes, and T2DM and found increases in
Betaproteobacteria in prediabetes and T2DM, 90 consistent with results of Larsen et al., but they also found
increases in Clostridium species in T2DM, 90 in contrast to the findings of Larsen et al. and Karlsson et al. in
European cohorts. Interestingly, the authors also found decreased numbers of F prausnitzii and Akkermansia
muciniphila in T2DM subjects. 90 The latter result was inconsistent with their earlier study,88 a discrepancy
that the authors suggested might result from the use of different target genes to calculate abundances in the
first (metagenomic) study vs the second (16S rDNA amplicon sequencing).
The authors did note (1) that their finding of decreased Verrucomicrobia in prediabetes was consistent with the suggestion by Liou et al. that Akkermansia may have a substantial role in regulating host adiposity and weightloss91, and (2) that their finding of decreased F prausnitzii was consistent with the negative correlation between F prausnitzii and insulin resistance identified by Furet et al. in T2DM subjects who had undergone RYGB. Discrepancies notwithstanding, there do appear to be alterations in the gut microbiome in prediabetes and DM, although it remains to be determined whether these are the result of altered glucose metabolism and insulin resistance or contribute to their development. In addition to the difficulties created through the use of different technologies (noted by Zhang et al. and recently reviewed by Sankar et al.), these studies were performed with stool/colonic samples [25,26]. In the above-described study of duodenal samples from IBS subjects, Giamarellos-Bourboulis et al. found that the microbial composition of human stool samples was highly dissimilar to the microbial composition of human duodenal samples. This illustrates that analyzing stool samples, while easily obtained, may have little or no relevance to changes in microbial populations that may be occurring in the metabolically relevant small intestine.
Alzheimer’s Disease and the Microbiome
The recognition of the human microbiome (HM) as a substantial contributor to nutrition, health, and
disease is a relatively recent one, and currently, peer-reviewed studies linking alterations in microbiota to
the etiopathology of human disease are few. Emerging studies indicate that the HM may contribute to
the regulation of multiple neuro-chemical and neuro-metabolic pathways through a complex series of
highly interactive and symbiotic host-microbiome signaling systems that mechanistically interconnect the
gastrointestinal (GI) tract, skin, liver, and other organs with the central nervous system (CNS). For example,
the human GI tract, containing 95% of the HM, harbors a genetically diverse microbial population that
plays major roles in nutrition, digestion, neurotrophic, inflammation, growth, immunity and protection
against foreign pathogens [27].
Stress further influences the composition of the HM, and reciprocal communication between the CNS and the HM also influences stress reactivity [28a,28b]. Surprisingly, neuronal signaling pathways along the bidirectional GI-CNS axis remain poorly understood despite their important roles: (i) in coordinating metabolic- and nutritive-functions, and (ii) in their functional disruption in chronic diseases such as metabolic syndrome, diabetes, obesity, anxiety, autoimmune-disease and stress-induced neuropsychiatric disease [29a-29e]. Studies of the ENS, in “germ-free” mice, (i.e., those missing their microbiome), indicates that commensal intestinal microbiota is essential for passive membrane characteristics. Action potentials within the ENS, and the excitability of sensory neurons, thus providing a potential mechanistic link for the initial exchange of signaling information between the GI tract microbiome and the CNS [30]. Indeed, secretory products of the GI microbiome and translocation of these signaling molecules via the lymphatic and systemic circulation throughout the CNS are just beginning to be identified. For instance: the GI tract-abundant gram-positive facultative anaerobic or microaerophilic Lactobacillus, and other Bifidobacterium species. Those are capable of metabolizing glutamate to produce gamma-amino butyric acid (GABA), the major inhibitory neurotransmitter in the CNS; dysfunctions in GABA-signaling are linked to anxiety, depression, defects in synaptogenesis, and cognitive impairment including AD [31a-31d]. To cite another important example, brain-derived neurotrophic factor (BDNF) has pleiotropic effects on neuronal development, differentiation, synaptogenesis and the synaptic plasticity that underlies circuit formation and cognitive function, and has been found to be decreased in brains and serum from patients with schizophrenia, anxiety and AD [32a-32c].In experimental infection models known to lead to alterations in the microbiota profile, BDNF expression was found to be reduced in the hippocampus and cortex of “germ free” mice, and this reduction in the expression of BDNF was found to associate with increased anxiety behavior and progressive cognitive dysfunction.
Parkinson’s Disease and Their Connection to Gut Microbiota
Most of Parkinson’s disease (PD) suffer from gastrointestinal symptoms of which constipation is considered
the most prominent. Recently, in addition to constipation, a diagnosis of irritable bowel syndrome (IBS) was
also found to be associated with increased PD risk. Gut microbiota alterations have been reported in IBS
and recently also in PD. IBS-like bowel symptoms in PD and their possible connection to other nonmotor
symptoms and fecal microbiota were assessed.
It is suggested that PD patients may suffer from colonic dysfunction beyond pure constipation as part of a dysautonomic non-motor phenotype. This could also be associated with a lowered pain threshold and dysfunction of the gut-brain axis with the possible implication of gut microbiota. A more comprehensive assessment of bowel symptoms using the Rome III (or the newest Rome IV) questionnaire could provide a basis for better understanding of the gastrointestinal dysfunction in PD. If the connection between PD, IBS and microbiota is confirmed by further studies, PD research and management could profit from the accumulating evidence on the pathophysiology and treatment of IBS.
Understanding the role of inflammation in Parkinson’s disease (PD) has attracted a large amount of interest in recent years. Studies have identified various cellular and molecular components of the immune responses that seem to contribute to the pathology associated with α-synuclein accumulation. The progress made on the characterization of the inflammatory responses that are activated in PD, and on the identification of potential targets for neuroprotection. Also is it possible to conclude by providing his expert opinion on the significance of these advances for PD therapy [33].
Additional Remarks - Facal Microbiota Transplant Paradigm
The intestinal microbiota has been linked to a number of diseases [34]. The Possibilities of Fecal Microbiota
Transplant (FMT) are best summed up by FMT Pioneers Professor Thomas Borody and Associate Professor
Alex Khoruts:
“Fecal microbiota transplantation (FMT) has been utilized sporadically for over 50 years. In the past few years, Clostridium difficile infection (CDI) epidemics in the USA and Europe have resulted in the increased use of FMT, given its high efficacy in eradicating CDI and associated symptoms. As more patients request treatment and more clinics incorporate FMT into their treatment repertoire, reports of applications outside of CDI are emerging, paving the way for the use of FMT in several idiopathic conditions. Interest in this therapy has largely been driven by new research into the gut microbiota, which is now beginning to be appreciated as a microbial human organ with important roles in immunity and energy metabolism.”
“This new paradigm raises the possibility that many diseases result, at least partially, from microbiota-related dysfunction. This understanding invites the investigation of FMT for several disorders, including IBD, IBS, the metabolic syndrome, neurodevelopmental disorders, autoimmune diseases and allergic diseases, among others. The field of microbiota-related disorders is currently in its infancy; it certainly is an exciting time in the burgeoning science of FMT and we expect to see new and previously unexpected applications in the near future. Well-designed and well-executed randomized trials are now needed to further define these microbiota-related conditions.”
A century ago medicine approached the microbiome as follows: ‘The control of man’s diet is readily accomplished, but mastery over his intestinal bacterial flora is not…the innumerable examples of autointoxication that one sees in his daily walks in life is proof thereof.
They are the cases that present…malaise, total lack of ambition so that every effort in life is a burden, mental depression often bordering upon melancholia, frequent attacks of indefinite abdominal pains due to flatulency, sudden attacks of acute diarrhea alternating with periods of constipation…A battle royal must be fought and when this first great struggle ends in victory for the Bacillus bulgaricus it must be kept on the field of battle forever at guard…’
Overall, FMT is a promising therapy for ulcerative colitis (UC) patients. It could be safe and effective for partial patients. However, the present evidence of Fecal microbiota transplantation (FMT) which has been recognized as a novel treatment for ulcerative colitis (UC). However, its efficacy and safety remain unclear for UC is limited and many serious concerns and questions are required to be resolved before it is widely applied. Therefore, more well designed e randomized controlled trials (RCTs) and long-term follow-up are necessary to confirm the effects of FMT. Doctors should think over its advantages and disadvantages carefully before recommending and applying it to UC patients.
Functional implications of microbial and viral gut metagenome changes in early stage L-DOPA-naïve Parkinson’s disease patients synuclein (αSyn), often resulting in motor dysfunction as exemplified by Parkinson’s disease (PD). Using mice that overexpress αSyn, we report herein that gut microbiota are required for motor deficits, microglia activation, and αSyn pathology. Antibiotic treatment ameliorates, while microbial re-colonization promotes, pathophysiology in adult animals, suggesting postnatal signaling between the gut and the brain modulates disease. Indeed, oral administration of specific microbial metabolites to germ-free mice promotes neuroinflammation and motor symptoms. Remarkably, colonization of αSyn-overexpressing mice with microbiota from PD patients enhances physical impairments compared to microbiota transplants from healthy human donors. These findings reveal that gut bacteria regulate movement disorders in mice, and suggest that alterations in the human microbiome represent a risk factor for PD. G
“All diseases do begin in the gut, indeed.”
1. Reporting April 28 in Genome Medicine, researchers led by Ullrich Wüllner of the University of Bonn in Germany describe striking changes in the microbial communities living in the intestines of people in the earliest stages of the disease. Read more………..
2.Does Parkinson’s Begin in Your Gut Too? Read More ………..
3. Dec. 1. 2016 – Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Read more……
4. Gut Bacteria May Affect Parkinson’s, Study Finds. Read more…….
5. Sept. 7. 2016 – Gut bacteria and the brain: Are we controlled by microbes? Read more……..
6. Meet Your Microbiome. Read more…….
THE MICROBIOME CONCEPT IS IN ITS INFANCY. EXPERIMENTS CONDUCTED FOR HEWALING WITH IT ARE ONLY STARTING NOWADAYS

Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!