Biography
Interests
Mamdouh Ibrahim Nassar1* & Emad Elzayat, M.2
1Faculty of Science, Biology-Entomology Department, Cairo University, Giza, Egypt
2Faculty of Science, Zoology Department, Cairo University, Giza, Egypt
*Correspondence to: Dr. Mamdouh I. Nassar, Faculty of Science, Biology-Entomology Department, Cairo University, Giza, Egypt.
Copyright © 2019 Dr. Mamdouh I. Nassar, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Cancer is a serious health problem and statistically, there are approximately 32.6 million cancer patients around the world. This Review is focusing on cytotoxic and antitumor activities of honey bee (Apis mellifera L.) venom. It is no doubt that cancer is one of the primary causes of death in the world. Complications connected with the use of chemotherapy and radiation therapy in cancer treatment might lower the effectiveness of such medication. So, using naturalistic yields in cancer treatment has become an important current topic. Therefore Bee venom (BV) has been suggested as an Apitherapy tool to be considered for various diseases including cancer. The bee venom has been found to have anticancer activities in different cancer cell lines involving breast, liver and prostate. However, the mechanisms action of BV and its toxicity on tumorigenic and nontumorigenic cells are poorly understood. BV is highly toxic to cancer cell lines and its mechanism action causes a cleavage of genomic DNA and inhibition of cell migration, indicating induction of apoptosis. Immunohistochemistry studies demonstrated that BV decreased the expression of Bcl-2 and P16. BV composed of many complex substances such as peptides (melittin, apamin, secapin, tertiapin, adolapin, and mast cell degranulating peptide). Melittin links to some tumor cells at a higher affinity than to healthy cells; anti- metastatic anti-invasive, and anti-angiogenesis impact which might earn future clinical research on its anti-tumor characteristics and therapeutic effects when used as a cancer diseases treatment.
Introduction
Order Hymenoptera (ants, bees, and wasps) contains over 115,000 species worldwide, [1,2] making it the
second largest order of insects. It contains the largest group of organisms on earth which possess a chemical
defense which is injected directly into the victim. Venoms in general are of particular interest to natural
product drug discovery due to the obvious pharmacological effects they have on their targets. According
to the Global Cancer Statistics published in 2015, there are approximately 32.6 million cancer patients
around the world in 2012 [3]. It is no doubt that cancer is one of the primary causes of death in the
world [4]. Over the last three decades, the 5-year relative survival rate for all type of cancers has increased
significantly, and this is partially due to the successful development of targeted therapy [4]. New studies
have reported that bee products have a prospective effect against cancer in vivo and in vitro [4]. Whereas
while using radio- and chemo- therapy to get rid of cancerous cells, also they hurt normal cells and cause
un-wanted side effects that restrict the treatment and effectiveness [4]. Honeybee venom (BV) has been
traditionally used with the hope to cure several diseases such as cancer, arthritis, and rheumatism [6-10]. BV
composed of many complex substances such as peptides (melittin, apamin, secapin, tertiapin, adolapin, and
mast cell degranulating peptide), enzymes (phospholipase A2, hyaluronidase, acid phosphomonoesterase,
lysophospholipase), active amines (histamine, dopamine, norepinephrine, serotonin), and other components,
which have a comprehensive pharmaceutical properties to some extent [5-10]. Recent studies have revealed
that the BV increases cytoplasmic Ca2+ and reactive oxygen species (ROS) and decreases mitochondrial
membrane potential, which enhance the levels of caspase-3, PARP, FAS, p53, p21 and Bax, and reduces
level of Bcl-2. The effects of BV on the DNA fragmentation are due to its ability to enhance the caspase-8
and caspase-9 through promoting caspase-3 activation [11-14]. Melittin, the principal active component of
BV, alone induced apoptosis in human leukemic U937 cells through reducing Bcl-2, NF-kB and increasing
caspase-3, [9,10]. Other studies showed that BV inhibited cell invasion and migration by suppressing
the MMP-9 activity and expression through inhibiting of NF-κB via p38 MAPK and JNK signaling
pathways in PMA-induced MCF-7 cells [11], and by suppressing MMP-2 and MMP-9 activity in mouse
skin fibroblast and myelogenous leukemia cell lines [12]. A recent study showed that BV and melittin
substantially decelerate capability of invasion and migration of breast cancer cells via inhibiting the EGFinduced
MMP-9 expression by blocking the NF-kB and PI3K/Akt/mTOR pathway [13]. The protein,
Ki-67, was suppressed by BV in SMMC-7721 cells [14]. The aim of the present review was to determine
therapeutic activities of Bee venom (BV) and its mechanism action when used as a cancer diseases treatment.
Cancer Causes
Cancer diseases are serious health problem where it is the cause of death mainly correlated with ageing
and lifestyle [4]. Interestingly, many observations have guided Researches to a novel hypothesis for cancer
mechanism. We should bear in mind, though, that in the majority of cancer cases we cannot attribute the
disease to a single cause. Cancer is caused by accumulated damage to genes. Such changes may be due to
chance or to exposure to a cancer causing substance. The substances that cause cancer are called carcinogens.
A carcinogen may be a chemical substance, such as certain molecules in tobacco smoke. The cause of cancer may be environmental agents, viral or genetic factors. From cancer causing factors related to work and living
environments include: asbestos fibers, tar and pitch, polynuclear hydrocarbons (e.g. benzopyrene), some
metal compounds and ome plastic chemicals (e.g. Vinyl chloride). Bacteria and viruses can cause cancer:
Helicobacter pylori (H. pylori, which causes gastritis), HBV, HCV (hepatitis viruses that cause hepatitis),
HPV (human papilloma virus, papilloma virus, which causes changes eg. Cervical cells) and EBV (Epstein-
Barr virus, the herpes virus that causes inflammation of the throat lymphoid). Radiation can cause cancer:
ionising radiation (e.g. X-ray radiation, soil radon) and non-ionised radiation (the sun’s ultraviolet radiation)
[15].
The chief, that cancer occurs just on those multicellular creatures whose have complex wound-healing abilities. The activation of oncogene occurs in normal physiology and non- cancer pathology ways and not just in cancer. Wounds stimulate oncogenes of some cells and the other release cytokines to induct stem cells to cure the wounds [16].
Bee Venom Therapy
Bee venom and is naturalistic toxin could be helpful as an anti-tumor factor through the overexpression of
DR3 and inactivation of NF-κB for the treating of lung tumor cells and drug resistant tumor cells [17].
[18] reported that BV inhibits proliferation of melanoma K1735M2 cells in vitro, as well as B16 melanoma,
a transplantable solid melanoma in C57BL/6 mice, invivo. The proliferation of K1735M2 cells in vitro
was inhibited by BV in a concentration- and time-dependent manner. The inhibition was indicated by the
arrest of the cell cycle at the G1 stage, as detected by flow cytometric measurements. Bee venom induced
apoptosis-like cell death as identified by histological observations and by DNA fragmentation. In the in
vivo study, BV was injected intraperitoneally into the mice 24 h after they had been inoculated with B16
cells and inhibition of the solid tumor was observed. Treatment with BV at concentrations of 1 or 5 mg/
ml decreased the viability of human lymphoma cell line HL-60 and human lymphocytes after 24h [19].
BV induced cell membrane lysis in HL-60 cells probably due to PLA2 present in the venom. BV induced
DNA fragmentation and micronuclei in HL-60 cells and also increased the expression of phosphate and
tensin homolog (PTEN), a tumor suppression protein, inducing cell cycle arrest in S phase, inhibiting
the proliferation of these cells. The molecular mechanisms of apoptosis induced by BV in human breast
cancer MCF-7 cells [20]. BV induced morphological changes and inhibited proliferation in a dose- and
time-dependent way in MCF-7 cells. Besides, BV induced reactive oxygen species (ROS) production and
dysfunction of mitochondria membrane potential, releasing cytochrome c, as well as an increase in the levels
of caspase-9 e Poly (ADP-ribose) polymerase (PARP), leading cells to apoptotic death. Furthermore, it
has been shown that BV induces DNA damage in these cells, as verified by the comet assay. The apoptotic
mechanism generated by BV on human cervical cancer Ca Ski cells. BV induced morphological changes
and decreased the percentage of viable Ca Ski cells in a dose- and timedependent manner. Flow cytometric
analysis demonstrated that BV induced the production of ROS, increased the level of cytoplasmic Ca2,
reduced mitochondrial membrane potential which lead to cytochrome c release, and promoted the activation
of caspase-3 followed by DNA fragmentation, leading to apoptosis. A decrease in the level of Bcl-2 (B-cell
lymphoma 2) and an increase in the levels of Fas, p53, p21 and Bax (Bcl-2-associated X protein) were also
observed. As demonstrated by [22] for MCF-7 cells, the also showed that BV promotes apoptosis of Ca
Ski cells through the mitochondrial pathway. BV therapy induces both caspase-dependent and caspase- independent apoptotic cell death through the stimulation of intracellular Ca (2+)- modulated intrinsic
death pathway in human bladder tumor cells [15].
Active Components of Bee Venom
BV is known for being composed of a complex mixture of active peptides, (melittin, apamin, secapin, tertiapin,
adolapin, and mast cell degranulating peptide) enzymes and amines [15,21]. It encourages membrane lysis
and prevents tumor cell proliferation, and enhances cancer cell apoptosis by a rise in reactive oxygen species
(ROS) and an increase in intracellular Ca2+. Melittin is effective against many cancer types involving
leukemia ,liver ,lung ,renal, prostate ,bladder and mammary cancer. It has many important functions such
as: calmodulin inhibitor [6], potent pore-forming factor, hyperactivates PLA2 in ras oncogene- transformed
cells [22], produces cell membrane lysis and apoptosis [23], acts in different cell signaling pathways [24].
Besides the antitumoral effect bee venom based drugs like peptides and melittin and it can be used to fight
cancer bee propolis. Propolis is a resinous material and one of the products of honeybees. It has been shown
that propolis has many actions, including anti-inflammatory, antibacterial, antiviral, imunommodulatory
and antiproliferative effects. A compound called caffeic acid phenethyl ester (CAPE), which is present in
propolis, has anti-cancer and antioxidant properties [25].
Application Strategies for Cancer Treatment
In an in vivo study, [7] reported that, while intravenously injected, bee venom importantly frustrated
mammary carcinoma metastasis significantly in murine injected also intravenously with this sort of cancer,
when contrasted to control murine [7]. Whist, no variations in metastasis formulation were noticed when the
venom was subcutaneously administered. Also, the tumor reduced in size while the venom was administered
intratumorally, and murine survived longer than control, suggesting that the in vivo venom action counts on
how the venom is injected. Bee venom shows a cytostatic impact in a dose- and time-dependent manner,
prevents proliferation and produces apoptosis of SMMC-7721 human hepatoma cells [23]. Three strategies
have been designed to reduce the adverse effects of melittin in not targeted tissues (1: coupling of melittin
to an antibody or a targeting component; (2: development of shielded pro-cytolytic melittin systems; and
(3: synthesis of melittin-transporting carriers [12]. Although melittin is the most studied and common bee
venom peptide, its development for clinical applications stays at most in preclinical phases. Furthermore,
flow cytometric analyses showed an accumulation of cells in the sub G1 phase of cell cycle in treated cells
compared to control. It was also demonstrated that BV treatment resulted in an increase in the expression of
Bax, a pro-apoptotic protein, and a decrease in the expression of Bcl-2, a protein that heterodimerizes with
Bax, suppressing cell death. Besides that, treated cells showed an upregulation of caspase-3 activity, a protein
that plays a role in the apoptotic pathway. The expression of COX-2 in NCI-H1299was lowcompared
to the control, and it is known that COX-2 is frequently up-regulated in tumors [26], so that selective
downregulation of COX-2 is an important strategy in the development of anti-tumor agents. In the
study, administration of an immunoconjugate containing a melittin-like peptide (peptide 101), improved
the survival of immune-deficient mice bearing subcutaneous human prostate carcinoma xenografts. The
specific antibodypeptide101 conjugate also significantly inhibited tumor growth compared to the controls:
unconjugated antibody or peptide alone. These new strategies can be used to decrease the non-specificity
of some toxins and also to increase the action potential, since the immunoconjugates showed a greater anti-cancer potential than the peptide alone. In the latest years, PLA2 isolated from BV has become of
great interest due to its great anti-cancer potential [26]. The adjuvant treatment with bee venom-sPLA2
and phosphatidylinositol-(3,4)-bisphosphate (PtdIns(3,4)P2) was more effective than any of the single
components in the blocking of tumor cell growth [25]. This adjuvant treatment had a synergistic effect
together with potent cell lysis. The authors suggest that the observed cytotoxicity is due to the disruption of
the membrane integrity, the abrogation of signal transduction and the generation of cytotoxic lyso-PtdIns
3,4 P2. They further demonstrated a reduction in the proliferation of the human cell kidney carcinoma
cell line (A498) employing the adjuvant treatment with sPLA2 and PtdIns 3,4 P2, associated with a
complete downregulation of PKB/Akt phosphorylation. The PI3-kinase/PKB/Akt pathway represents a
central survival-related signal transduction pathway and its activation enhances cell survival and promotes
tumor invasion [27]. Furthermore, treated cells exhibited a decrease of the epidermal growth factor receptor
(EGFr). The tumor lysates formed after treating the cells with bv-sPLA2 and PtdIns (3,4) P2 enhanced
the maturation of immunostimulatory human monocyte-derived dendritic cells. Such tumor lysates, which
represent complex mixtures of tumor antigens showing potent adjuvant properties, meet all the requirements
of a tumor vaccine for application in cancer immunotherapy [28].
Antitumor Bee Venom Melittin
Considering the venoms produced by arthropods, bee venom (BV) is the most studied regarding its anticancer
activities, due mainly to two substances that have been isolated and characterized: melittin and
phospholipase A2 (PLA2). Although melittin and PLA2 are the two major components in the venom of
the species Apis mellifera and many studies have been published describing their antitumoral effects [28],
BV is a very complex mixture of components that may cause other physiological effects. The first study was
published by Havas in 1950 and, after 30 years, other groups started to carry on interesting studies about
the cytotoxicity of bee venom upon tumor cells. Due to the promising effects found, publications have
been constantly growing, showing not only the effects of BV in tumor cell lines, but also characterizing the
signaling pathways through which the venom inhibits cellular proliferation, besides many interesting in
vivo studies. Besides melittin and PLA2, other important components are histamines, catecholamines and
polyamines. Melittin is by far the peptide with the greatest antitumor activity isolated from BV, acting in
different ways upon the physiology of cancer cells. Melittin is a small and amphiphilic peptide containing
26 amino acid residues and is the principal toxin derived from the venom of the bee, Apis mellifera. The
sequence of melittin is Gly-Ile-Gly-Ala- Val-Leu-Lys-Val-Leu-Thr-Thr-Gly-Leu-Pro-Ala-Leu-Ile-Ser-
Trp-Ile-Lys-Arg-Lys-Arg-Gln-Gln [29]. Melittin exhibits anti-microbial activities and pro-inflammatory
effects [30], besides inducing perturbations in the cell membrane and damage to enzyme systems [31,32].
Several cancer cells, including leukemia, renal, lung, liver, prostate, bladder, and mammary cancer cells, can
be targets of melittin [25]. [22] has shown that melittin is capable of binding calmodulin, which has a role
in cellular proliferation. [33] also showed that melittin is one of the most powerful inhibitors of calmodulin
activity and, as such, is an inhibitor of cell growth and clonogenicity of human and murine leukemic cells
[29,33,34]. Melittin inhibits the melanotropin receptor in M2R melanoma cell membranes [35]. Other
studies suggest that melittin acts in the same manner as poreforming agents, killing malignant cells [36,37].
Most recent studies have shown that melittin kills tumor cells by apoptosis through several cancer cell death
mechanisms, including the activation of caspase and matrix metalloproteinases (MMP) [38,39]. Besides
the above-mentioned effects, melittin also leads to cell death by other means. [40], Considering the venoms produced by arthropods, bee venom (BV) is the most studied regarding its anti-cancer activities, due mainly
to two substances that have been isolated and characterized: melittin and phospholipase A2 (PLA2).
Although melittin and PLA2 are the two major components in the venom of the species Apis mellifera
and many studies have been published describing their antitumoral effects [28], BV is a very complex
mixture of components that may cause other physiological effects. Melittin is a small and amphiphilic
peptide containing 26 amino acid residues and is the principal toxin derived from the venom of the bee,
Apis mellifera. [41] also reported that melittin, as a PLA2 activator, increased the calpain activity and cell
necrosis in the hepatocellular carcinoma cell lines N1S1 and McA-RH7777 [41]. Melittin can be effective
against leukemic cells, KG1a, CEM, and CEM/VLB100, which are relatively resistant to tumor necrosis
factor a (TNF-a), a cytokine that activates cell death [42]. This is because melittin can activate low levels of
cPLA2 activity in the KG1a cell line. Furthermore, melittinmediated cytolysis of U937 human monocytic
leukemia cells is associated with the transient activation of endogenous phospholipase-D, which has been
suggested to participate in an uncharacterized signal transduction pathway involved in the permeabilization
of cancer cell membranes [43]. Melittin and a fragment of a melittin-conjugated hormone receptor (e.g.,
hecate) were shown to have an anti-tumor effect in ovarian and testicular tumors. The melittin fragment
(hecate) conjugated to a 15-amino acid beta-chain of human chorionic gonadotropin (hCG) shown that the
conjugates selectively destroys ovarian cancer cells (OVCAR-3) in vitro and OVCAR-3 cells engrafted in
nude mice models in vivo [44]. In a group of animals treated by hecate-hCG-ß, tumor volume expressed as
a percentage of increase was 199 _ 18.57% when compared with control animals (263.0 _ 21.72%). In vitro
and in vivo studies with the melittin/avidin conjugate [38]. In vitro study, the conjugate presented a great
cytolytic activity in DU 145 prostate cancer cells and SK-OV ovarian cancer cells that exhibited high MMP-2
activity. Besides, the conjugate showed low cytotoxicity in normal cells with low MMP-2 activity in vitro. In
vivo, the tumors injected with the complex melittin/avidin were maintained with a smaller size comparing to
non-treated tumors, indicating the great potential of this treatment in the fight against cancer. A significant
antineoplastic effect was detected on the transplanted tumor in nude mice after an intratumoral injection
of Ad-rAFP-Mel. An AdrAFP-Mel infection markedly induces cellular apoptosis, and Fas expression on
Bel-7402 cells. They suggested this to be a possible molecular mechanism for the antitumorigenecity of
AdrAFP-Mel even though more studies will be needed [45]. The effects of BV in NCI-H1299 lung cancer
cells and verified that cells treated with 10mg/ml of venom for 24 h exhibited morphological changes typical
of apoptotic cells, which was confirmed by terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) assay, DAPI staining assay and DNA fragmentation detected via agarose electrophoresis [46].
A solution to increase melittin efficiency against tumors [47]. Tumor-specific antibodies can be used to
target melittin to tumor cells. Melittin, at concentrations above 0.075mM, increased the intracellular Ca2
via L-type Ca2 channels, without evoking Ca2 release from stores, in MG63 human osteossarcoma cells in
a concentrationdependent manner [48]. At concentrations of 0.5 and 1 mM, melittin killed 33% and 45%
of the cells, respectively, through apoptosis. The cytotoxic effect of 1 mMmelittinwas completely reversed by
pre-chelating cytosolic Ca2 with BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N0,N0-tetraacetic acid),
suggesting that apoptosis was due to an increase in intracellular Ca2. The combination of melittin with
TRAIL may be a promising therapeutic approach in the treatment of TRAIL-resistant human cancer.
Screened for HCC cell lines (hepatocellular carcinoma) with high expression levels of Rac1 (Rasrelated C3
botulinum toxin substrate 1) to study the relationship between the inhibitory effect of melittin on HCC
metastasis and the Rac1-mediated signaling pathway both in vitro and in vivo [49] and found that Rac1
plays a crucial role in the control of HCC cell motility and metastasis. Melittin prevents HCC metastasis via inhibition of Rac1. Melittin inhibited cell motility accompanied by a decrease in Rac1, ERK (extracellular
signal-regulated kinase), and JNK (c-Jun N-terminal kinases) activity, suggesting that melittin acts through
the suppression of Rac1-dependent pathways. In addition, the lung metastasis rate was significantly decreased
in the melittin-treated nude mouse model LCID20. However, the authors showed that administration of
high doses of melittin in vivo has its side effects, particularly liver injury and hemolysis. Considering that
HCC T.E. 502 usually develops in a background of chronic liver injury and impaired liver function, caution
will be required in the clinical application of melittin [50]. Finally, the authors commented that a mutation
of Val 5 to Arg, Ala15 to Arg, and deletion of Leu15 in melittin significantly reduces its adverse side effect
of hemolysis, but retains its antibacterial effect [49], showing that there are ways to overcome the toxic
effects of melittin in the organism in order to perform future clinical trials. The effect of melittin in human
leukemic U937 cells and the underlying intracellular signal transduction pathways involved in regulating
apoptosis [51]. Melittin induced a dose-dependent inhibition of the proliferation in U937 cells. After 48
h of treatment with more than 2mg/ml melittin, U937 cells exhibited morphological characteristics of
apoptosis, including cell shrinkage and nuclear condensation. These results suggest that melittin-induced
apoptosis contributes to the decreased proliferation of U937 cells. This apoptotic response was associated
with the upregulation of Bax and caspase-3 activation and downregulation of Bcl-2 and IAP (inhibitor
of apoptosis) family members. Moreover, the inactivation of Akt displayed by cells treated with melittin
also has an important role in the apoptosis process observed in these cells. In contrast, [52] showed that
melittin-induced apoptotic death in human melanoma A2058 cells was by a caspase-independent manner,
through generation of ROS and subsequent disruption of mitochondrial membrane potential transition,
followed by the release of AIF (Apoptosis Inducing Factor) and EndoG (Endonuclease G) into the nucleus.
Besides that, the role of Ca2 in cell death promoted by melittin was well established, once incubation of
cells under calcium-free conditions effectively diminished BV-induced apoptosis. And treatment of cells
with ouabain (a Na/K ATPase inhibitor) significantly blocked BV-induced cell death in human melanoma
A2058 cells. Another mechanism of the melittin anti-tumor action was recently proposed. Melittin inhibited
the enzymatic activity of matrix metalloproteinase-9 secreted by PMAinduced Caki-1 (renal carcinoma)
cells. MMP-9 plays an important role in the invasion and metastasis of cancer cells, and melittin inhibited
the levels of phospho-ERK and phospho-JNK, affecting the levels of AP-1 and NF-kB (nuclear factorkB),
which, in turn, led to suppression of MMP-9 expression [53]. Some recent studies have shown the
anti-cancer potential of melittin using nanocarriers to deliver this cytolytic peptide specifically to tumor
cells. Incorporated the nonspecific peptide melittin into the outer lipid monolayer of a perfluorocarbon
nanoparticle which revealed that a dramatic reduction of tumor growth without any apparent signs of
toxicity in mice [27].
Bee Venom Based Drugs
New peptides have been purified from bee venom and tested in tumor cells, exhibiting promising activities in
the treatment of cancer. Some of these interesting molecules are the lasioglossins isolated from the venomof
the eusocial bee Lasioglossum laticeps, which exhibited potent anti-microbial activity against both Grampositive
and Gram-negative bacteria, low hemolytic and mast cell degranulation activity, and a potency
to kill various cancer cells in vitro [50]. A breakthrough in use of venom-peptide came to light after the
development of captopril, an anti-hypertensive drug [54]. Captopril, an analogue of dipeptide Ala-Pro
from snake Bothrops jararaca effectively bind to the active site of Angiotensin- converting enzyme (ACE). ACE a key enzyme of renin- angiotensin system that converts angiotensin I to an active vasoconstrictor
angiotensin II which regulates the volume of fluids in blood. Captopril an active ACE inhibitor is used in
the treatment of hypertension. Following captopril footsteps, a tri-peptide Phe-Ala-Pro analogue enalapril
was also developed [54]. Captopril was approved for its use in 1981, and since then many venom-peptide
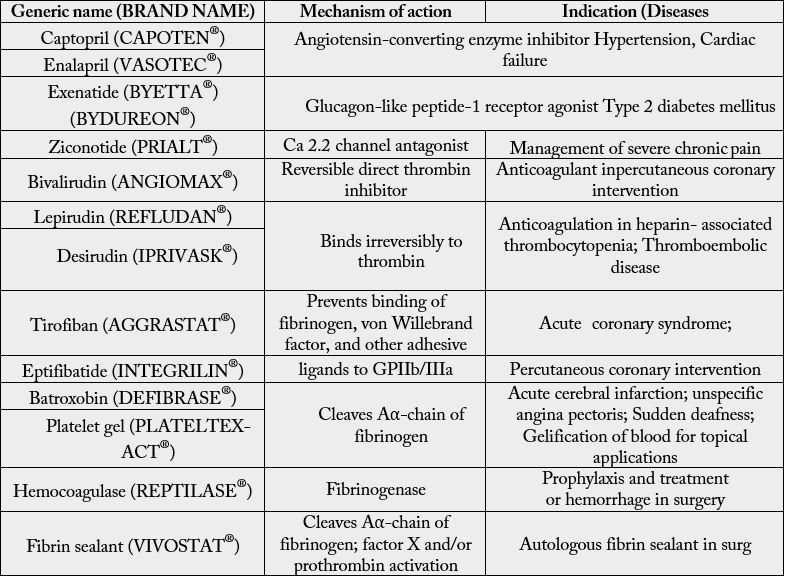
or venom-peptide analogues have been tested for various disease with few success (Table 1) [55]. Table 1
depicts various venom-based drug brands in the market today and its application against various disease
and its application against various disease conditions along with mechanism of actions. Many technical
advances during last decade have exemplified the importance of venom-peptide in drug discovery. Today,
using advanced proteomics and genomics approach it is possible to isolate and characterize the potential
anti- cancer peptides from venom pool. Further, structural analysis of isolated peptides and its interactions
with protein or target molecule has revealed specific amino-acid domains that exhibit anti-proliferative
effects, for example- importance of RGD domains in peptide including disintegrins family. In harmony
RGD sequence presents in most of the disintegrins isolated from snake species and provides a structural
scaffold for interactions with transmembrane receptor integrins (importance of disintegrins in anti- cancer
therapy is discussed later in this review). Structural modifications of such domain lead to increase stability
and elimination of liable peptide bonds that may comprise a peptide to enzymatic degradation. Venombased
peptides being small and easily modified, the prospect of using them or their analogues in cancer
therapy is promising. [56] investigated the possible growth-inhibiting effects of bee venom applied alone or
in combination with a cytotoxic drug, bleomycin, on HeLa and V79 cells in vitro. The adjuvant treatment
caused a dose-dependent decrease in cell survival due to DNA damage, suggesting that BV might find a
therapeutic use in enhancing cytotoxicity of the antitumor agent bleomycin.
Bee Venom and Immune Modulation
Immune reaction represents the primary defense system against non-self components including cancer cells
in an organism. In brief, the initial response to non-self components is mediated by innate immune response
cells such as natural killer cells (NK) and antigen presenting cells (APC) followed by adaptive immune
response mediated by T cells and B cells. Snake, bee, and wasp venoms are known to enhance both innate
and adaptive immune response in parallel to inducing a cytotoxic effect within the cells [57]. In harmony
some studies, bee venom is known to the by-pass innate immune system and directly influences T cell
activity or adaptive immune system [57]. Venom peptides such as cobra venom factor, cobra toxin, PLA2,
melittin from snake Naja naja atra were observed to enhance NK cells activity by increasing the production
of cell stress signaling protein interferon-g in immune-suppressed mice (IFN-g) [58]. In the same study,
authors have also observed suppression of T cell proliferation especially CD8 T cells through inhibition of
NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) [58]. In contrast, bee venom PLA2
and melittin are reported to increase T cell response by increasing synthesis of cytokines IL-1 and TNF-a
on monocytes [8].
Mechanism of Cancer Invasion
Compared to normal cells, cancer cells have the ability to circumvent the cell cycle checkpoint, responsible
for maintaining intracellular balance in vivo [59]. Hallmarks of cancer include sustaining proliferative
signaling, evading growth suppressors, activating invasion and metastasis, enabling replication immortality,
inducing angiogenesis, and resisting cell death. Besides, there is the introduction of two emerging hallmarks
including deregulating cellular energetics and avoiding immune destruction [60]. When normal cells acquire
the sustaining proliferative signaling, they will enable to get other hallmarks to become tumorigenic. So an
ideal anti-cancer drug would be able to inhibit and/or block any one or some of the hallmarks.
Although the multistep process of cancer development is divided into three physiological stages, i.e., initiation, promotion, and progression of cancer, the distinction between the three stages in the dimension of time is artifactual. In a leading edge review on cancer by Hanahan and Weinberg, authors discuss six important hallmarks of cancer that provides a logical framework for understanding the chronic process of cancer [60]. Metastasis of cancer cells is the leading cause of increased mortality in cancer patients. As the tumor grows slowly (usually less than 2mm3), the tumor cells are in a resting non-metastatic state at this time. In a sustained hypoxic environment, tumor cells need to get more nutrients and oxygen to survive and proliferate. Then tumor cells increase cellular hypoxia inducible factor (HIF) transcription, thereby increasing blood vessel secrete growth factors (such as VEGF-A, VEGF-C) and chemokines (such as TNFα) to active endothelial cells. These growth factors stimulate blood vessels and lymphatic vessels to up-regulate the expression of specific integrins, such as αvβ3, α1β1, and α5β1, etc. Then these integrins have the ability to recruit some matrix metalloproteinases (MMPs, such as MMP2, MMP9) to degrade the basement membrane of vessels, thereby promoting endothelial cell migration and remodeling to form new blood vessels and lymphatic vessels. The new blood vessels not only provide nutrition to the tumor cells to continue to grow but also transfer the metabolic waste. Meanwhile, the blood vessels and lymphatic vessels provide a path for the local and distant metastasis of cancer cells [16]. However, only about 0.01% of cancer cells are capable of entering the circulation of metastasis to spread to distant sites, but this process is fatal for most cancer patients [61]. Initiation of metastasis begins with migration and invasion of cancer cells from the primary tumor into the surrounding tissues. To invade tissues, cancer cells undergo a pathophysiological transformation involving changes in the membrane characteristics, the process known as epithelial-mesenchymal transition (EMT) [62]. This process is followed by migration and invasion of cancer cells into the lymphatic system, and into the secondary tissue. To successfully produce a secondary tumor, cancer cells again transform back into the epithelial cell by mesenchymal-epithelial transition (MET). MET is required for anchoring of cancer cells to the surrounding tissues. Thus, the process of cancer cell metastasis is governed by many factors such as growth factors (basic fibroblast cell growth factor (bFGF), vascular epithelial growth factors (VEGF), membrane ion channels, cytokines, cell adhesion molecules, and extracellular matrix (ECM) [62].
Antitumor and Future Prospects
Initial interaction of venom peptides with the target molecule is the first and foremost step which plays a key
role in venom-peptide induce anti-cancer activity. Many of the examples used in this review emphasize the
importance of this initial step. This interaction is guided either by the polarity of a molecule or by specific
pharmacophore domain. Followed by initial interactions, peptides tend to exhibit their effects mostly by
membrane interactions, although many other mechanisms such as intracellular peptide-protein interaction,
peptide-DNA interactions are still existing. Peptide based targeted therapy has gained momentum in the
last two decades. Smaller size and tumor penetrating ability makes peptides an ideal choice for targeting
cancer cells. Among many, peptide based anticancer drugs in market three peptides Leuprolide, Octreotide
and Goserelin have reached a global sale of 1 billion US dollar per year [71]. Today, a wide range of synthetic
peptides have been tested for their anticancer abilities among which some are in clinical trials. Synthetic
peptides such as Cilengitide, IM862, ATN 161, and angiotensin- [63], are being tested against various types
of cancers. Synthetic peptides with cancer cell specificity can also be used to deliver a cytotoxic drug, or as
hormone antagonist, or even as a vaccine in reducing or stimulating immune reactions within the system.
Recent studies on molecular aspects of cancer development and progression have also offered prospects
in both identifying potential targets and designing potential ligands [64]. Many of potential anti-cancer
peptides such as stimuvax, primovax, melanotan are in clinical trials for elucidating efficiency, bioavailability,
and metabolism [63,65]. Therefore, Venom based peptides have a wide range of application in modern
biology from diagnostic to the treatment of the disease [5]. For example, Chlorotoxin analog drug BLZ 100 tagged with a fluorescence dye is able to lights up only cancer cells in brain tumor so that it can be
precisely excised from the brain [5]. BLZ-100 is also known as “Tumor paint” is currently in phase 1b
clinical study. Furthermore, advancement in proteomics and genomics approach has made possible to isolate
and characterize the potential anticancer peptides from venom pool. High throughput screening using mass
spectrometry is very useful to venom characterization as it can read low concentration peptides along with
generating mass datasets that could be analyzed further. Similar sequencing RNA isolates from venom
glands will provide generate the pool of expressed proteins and peptide database. Peptidomimetic approach
promotes future use of peptide- based drugs by (i) increasing the stability of peptides against chemical
degradation and enzymatic degradations leading to increasing lifetime within the biological system; (ii)
decreasing the size of peptides making the molecule smaller and easily accessible for interaction; and (iii)
by managing the electrostatic charge distribution and polarity of peptide that is important for peptide
interactions and to make cancer treatment affordable to patients in order to create new design and discovery
of anti- cancer drugs [3,66-68].
Conclusions
Bee venom (BV) is the most investigated venom among the other arthropod venoms because of its antiproliferative
potential. In fact, melittin, a major component of BV has been shown to possess greater
antitumor activity. However, as reported earlier other components of the BV may be responsible for the
biological activities of it. The anti-proliferative activity of BV involves in apoptosis because BV induced DNA
fragmentation in cells, so it is indeed, the antiproliferative activity of BV involves induction of apoptosis and
suggested that BV displays its pharmacological effects via induction of apoptotic pathway rather than necrosis.
This suggests that the BV suppresses cell proliferation using another pathway apart from the suppression of
DNA topoisomerase I. Immunohistochemical staining of the BV treated cell showed increased P16 levels
indicating confirming its antiproliferative activity. BV caused decreased expression of Bcl-2, Ki-67, Cyclin
D1, and P53 in HeLa and HT29 cells, indicating the apoptotic and apoptosis-promoting effects of BV.
Interaction of venom peptides with the target molecule is the first and foremost step which plays a key role in venom-peptide induced anti-cancer activity. Many of the examples used in this review emphasize the importance of this initial step. This interaction is guided either by the polarity of a molecule or by specific pharmacophore domain. Followed by initial interactions, peptides tend to exhibit their effects mostly by membrane interactions, although many other mechanisms such as intracellular peptide-protein interaction, peptide-DNA interactions are still exist. The targeted therapy drug with the specificity on certain molecule determines the limitation of its use, for example trastuzumab can only be used for HER2-positive breast cancer which occupies about 20% of breast cancer patients. Drugs derived from venom are no exception. Currently, venom-based drugs such as chlorotoxin and integrin αvβ3 drugs are used mainly in brain tumor and cancer with overexpressed αvβ3, respectively. Understanding mechanism of action of venom-peptides helps to curate a “staple peptide” with increased specificity in various types of cancer cells. As the molecular interaction of each venom peptide may vary, each peptide needs to be evaluated for its therapeutic potential. This review on venom peptides in cancer therapy fortifies our current understanding of their molecular mechanism of action and paves the way for better utilization of venom-based drugs.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!