Biography
Interests
Maura Rojas-Pirela1,4*, Luis Ramírez2, Marianela Zambrano2, María José Peña-Vera2, Cristóbal Lárez- Velásquez3* & Elizabeth Pérez-Pérez2*
1Laboratorio de Enzimología de Parásitos. Departamento de Biología, Facultad de Ciencias, Universidad de Los Andes, Mérida, Venezuela
2Laboratorio de Análisis Biotecnológico y Molecular (ANBIOMOL) “Prof. Guillermo López Corcuera”, Facultad de Farmacia y Bioanálisis, Universidad de los Andes, Mérida, Venezuela
3Laboratorio de Polímeros, Departamento de Química, Facultad de Ciencias, Universidad de Los Andes, Mérida, Venezuela
4Instituto de Biología, Pontificia Universidad Católica de Valparaíso, Valparaíso, Chile
*Correspondence to: Dr. Maura Rojas Pirela, Departamento de Biología, Facultad de Ciencias; Elizabeth Pérez-Pérez, Laboratorio de Análisis Biotecnológico y Molecular (ANBIOMOL) “Prof. Guillermo López Corcuera”, Facultad de Farmacia y Bioanálisis; Cristóbal Lárez-Velásquez, Laboratorio de Polímeros, Departamento de Química, Facultad de Ciencias, Universidad de Los Andes, Mérida, Venezuela.
Copyright © 2019 Dr. Maura Rojas Pirela, Elizabeth Pérez-Pérez, Cristóbal Lárez-Velásquez, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Sinusitis is a symptomatic inflammation of the paranasal sinuses and nasal cavity affecting millions of people in the world. This condition can be caused by different factors, including fungi, viruses and bacteria. In this work different bacterial strains isolated from patients diagnosed with chronic sinusitis were characterized. The identification of these isolates was made by morphological, biochemical and microbiological studies. The isolates belonged to the family Enterobacteriaceae. At the genus level, the genera Enterobacter, Klebsiella and Escherichia coli were found. Studies of resistance to different antimicrobial compounds showed that the isolates were simultaneously resistant to at least four of the commercially available antibiotics. Several of these antibiotics are beta-lactams and derivatives, such as amoxicillin, which are the antibiotics most used for the treatment of acute and chronic sinusitis. In addition, these isolates were found to be tolerant at NaCl concentrations of up to 5%.
Introduction
Sinusitis is an infection that causes inflammation of the mucosa of the paranasal sinuses. Different factors
promote the development of sinusitis. However, most of the time it is caused by pathogens such as viruses,
fungi and bacteria [1,2]. This inflammation caused for the colonization of sinus tissues by pathogenic
microorganisms interferes with air drainage, causes excessive accumulation of mucus and difficult the
respiratory process through the nose. The symptoms of this disease can last 3 or more than 8 weeks, depending
on the type of sinusitis (if it is acute or chronic) [2]. Sinusitis is one of the most common respiratory diseases,
having a significant global impact. Only in the case of the United States, it is estimated that sinusitis
affects 31 million patients annually. Like other conditions such as rhinitis, this condition is characterized
by significantly reducing the quality of life of the individual [3]. On the other hand, this condition has a
significant economic and social impact. This affection causes significant direct medical expenses and loss
of work and school days, a reduction in productivity of the workplace and school learning [4]. Different
methods are applied for the diagnosis of sinusitis. However, if the condition does not respond to treatment or
worsens, tissue cultures and/or sampling of nasopharyngeal tissue can help determine the cause. In the case
of bacterial sinusitis, it is caused by different bacterial species. The species Staphylococcus aureus, Streptococcus
pyogenes, Streptococcus pneumoniae, Moraxella catarrhalis, Entobacter spp, Klepsiella pneumonia, Escherichi coli,
Pseudomonas aeruginosa, among others, are the bacterial species associated with sinusitis [5-7]. Nasal saline
irrigation and antibiotics are the main tools used to control and treat sinusitis. The application of saline nasal
solutions is used as an alternative to reduce the use of antibiotics [8].
Recent reports have shown the emergence of antibiotic resistant bacteria in sinusitis and their increasing rate of multidrug-resistance (MDR) [5,6]. In the case of the antibiotics, in addition to the empirical therapy of these and the indiscriminate use of these antimicrobial compounds as a simple palliative of the symptoms of sinusitis, it has led to an increase in the appearance of strains resistant to multiple antibiotics [5,6]. Due to the increased resistance to antibiotics in several bacterial strains isolated from the upper respiratory tract, the aim of this study was to determine the pattern of resistance to multiple antibiotics and salt-tolerance of the bacteria coming from patients diagnosed with chronic sinusitis.
Materials and Methods
Studies were conducted on 11 patients with chronic sinusitis. Samples of nasopharyngeal exudates were
collected with sterile cotton swabs from all 11 patients diagnosed. Standard microbiological procedures
were performed for identification of the bacteria causing chronic sinusitis [9-11]. In order to identified the
isolates under study and achieve a preliminary taxonomic location at the group or family level, the following
biochemical tests were performed, according to procedures previously described [9-11]: Hugh and Leifson medium, nitrate reduction, MIO (Motility, Indol, Ornithine), oxidase test, MacConkey Agar and urea
hydrolysis.
Resistance to antibiotics was studied in LB medium prepared with commercially antibiotics purchased on drugstores. Antibiotics in tablet presentation were: Ampicillin (Amp) 500mg, Kanamycin (Kan) 500mg, Amoxicillin/Clavulanic (Amc) acid 500mg, Cephalothin (Cf) 1g, Cefadroxil (Cfr) 500mg, Ciprofloxacin (Cxm) 500mg, Gentamicin (Gm) 80mg. A stock solution was prepared of each of them and employed to prepare MLD medium plates (25mL) with the following concentrations of each antibiotic: 25, 50, 100 and 200μg/mL. The strains were then inoculated using chopsticks and incubated at 37°C, taking readings at 24 and 48 h of incubation. Each experiment was performed in triplicate.
The strains salt-tolerance to sodium chloride (NaCl) was evaluated employing plates of LB medium supplemented with four different concentrations of NaCl (2.5-15% m/v) which were subsequently incubated at 35°C for 24 h.
Results
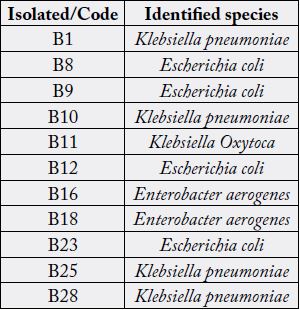
The results of biochemical and microbiological studies of the strains isolated from all 11 patients diagnosed
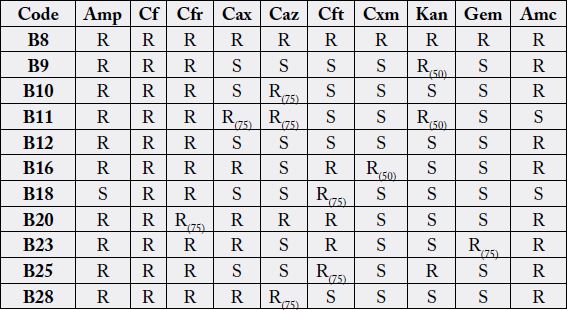
with chronic sinusitis are presented in Table 1. Studies of antibiotic resistance were carried out using
commercial antibiotics prepared at different concentrations (see materials and methods). Table 2 shows the
results of the resistance studies to different antibiotics of the different strains identified. Results of the strain
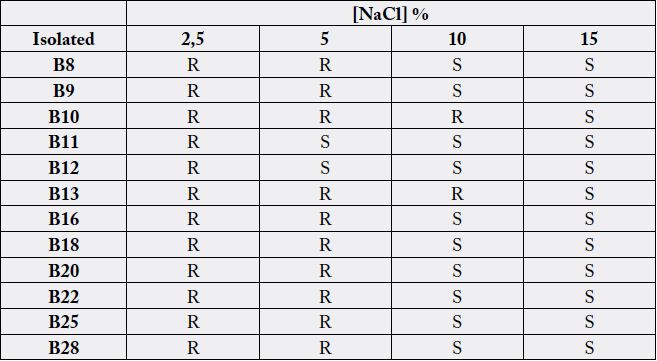
resistance to different concentrations of NaCl are presented in Table 3.
Discussions
A total of 11 patients were evaluated in our study. The results of biochemical and microbiological studies
showed that all 11 characterized strains were located taxonomically within the group of Enterobacteriaceae.
Based on the results presented in Table 1, it was determined that the isolates studied belong to the Klebsiella
pneumoniae, Escherichia coli, Enterobacter aerogenes and Klebsiella oxytoca. Similar bacterial species have
also been identified in other studies conducted with strains isolated from patients diagnosed with chronic. In general, all identified bacterial genera have showed greater resistance to Amp, Cf, Cfr, Cax, and Amc
antibiotics, while they were sensitive to Cxm, Kan and Gem. These isolates showed antibiotics resistance
patterns similar to those reported in the present work.
In some studies, multidrug-resistant (MDR) isolates were defined resistant to three or more antibiotics [6]. In our case, all the bacteria identified were resistant to more than three antibiotics (Table 2). Some of these isolates were resistant to 10 antibiotics simultaneously. In some antibiotics these isolates have the ability to grow to concentrations as high as 100µg/ml; in other cases, they were resistant only to concentrations lower than 50-75µg/ml (Table 2). Some antibiotics to which they showed resistance belong to beta-lactams of 1st, 2nd and 3rd generation [13]. This could be indicative that these isolates have an extended spectrum of β-lactamases (ESBLs) enzymes, encoded by genes described predominantly on plasmid, that are common among Enterobacteriaceae, in particular Klebsiella pneumonia and Escherichia coli. These enzymes are produced by a variety of Gram negative bacteria which confer an increased resistance to commonly used antibiotics [14-16]. Antibiotics to which these bacteria show resistance, as Amp y Amc, are some of the antibiotics worryingly used for the treatment of acute or chronic sinusitis [17,18].
Infections with ESBL-producing organisms are mostly hospital-acquired. However, in our study, none of the patients had nosocomial infections. The risk factor for the spread of ESBLs bacteria has probably been the indiscriminate use of antibiotics during the treatment applied for these infections. Antibiotic resistance is increasing in bacteria causing chronic sinusitis, especially to beta-lactam antibiotics, which was observed among Gram negative bacteria in our study.
On the other hand, saline solutions are usually administered as adjuvant therapies to attenuate the symptoms of chronic sinusitis as well as to treat and prevent upper respiratory tract infections, especially in children [19,20]. The beneficial effects of nasal applications of saline solutions is attributed to the direct cleaning of the nasal mucosa, removal of inflammatory mediator, inhibition of the bacterial growth and removal of antigens responsible for allergic reactions, favoring resolution of respiratory infections of the upper respiratory tract and rhinitis. The efficacy of nasal irrigation with these solutions is multifactorial [21].
Evaluation of the strains resistance to different concentrations of NaCl (Table 3) showed that most of the isolates were resistant at least at a concentration of 5% NaCl, which is far above the concentration of commercially available salt solutions used for the treatment of sinusitis [22,23]. In different studies it has been shown that NaCl has an antibacterial effect. High concentrations of NaCl affect the mobility, biofilm formation and growth and expression of genes in some Gram negative bacteria, as Pseudomonas aeruginosa [24]. Tolerance to NaCl concentrations found in this research could indicate that these isolates could have an unknown mechanism to maintain the osmotic balance between the cytoplasm and the surrounding medium [25].
These bacteria present simultaneously mechanisms of resistance to antimicrobial compounds and NaCl. Both groups of substances are involved in the treatment of sinusitis. According with these results, it could be suggested that it is unlikely that infections caused by these isolates can be controlled by beta-lactam antibiotics or alternative treatments, such as saline nasal irrigation employing a saline concentration of less than 5%.
Conclusions
Bacterial isolates from patients diagnosed with sinusitis, belonging to the Enterobacteriaceae family, shown
resistance to at least 4 commercially available antibiotics. They shown resistance to beta-lactamics and
derivated, as amoxicillin, which are the antibiotics most employed for the treatment of acute and chronic
sinusitis. Additionally, multi-drug-resistance was also observed in these bacterial isolated.
Furthermore, the majority of the bacteria studied in this work shown resistance to high concentrations of NaCl, including NaCl concentrations higher than commercial saline solutions prescribed for the treatment of acute and chronic sinusitis.
Bacterial strains evaluated in this work seem to have developed mechanisms that allow them to survive treatments with antibiotics and/or saline solutions commonly prescribed in the treatment of sinusitis.
Acknowledgements
None
Conflicts of Interests
The authors declare not conflict of interests
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!