Biography
Interests
Mailson Batista Fernandes1* & Aljerry Dias do Rêgo1,2
1Faculty of Medicine, Federal University of Amapá, Amapá, Brazil
2Department of Gynecology, Faculty of Medicine, Federal University of Amapá, Amapá, Brazil
*Correspondence to: Dr. Mailson Batista Fernandes, Faculty of Medicine, Federal University of Amapá, Amapá, Brazil.
Copyright © 2019 Dr. Mailson Batista Fernandes, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Polycystic ovary syndrome (PCOS) is one of the most common endocrine diseases in women
in the menacme and involves complex pathophysiology, with genetic, metabolic and hormonal
factors acting synergistically. Its endocrine-metabolic characteristics have been related to changes
in thyroid physiology. PCOS and autoimmune/hormonal thyroid disease are disorders related to
increased cardiovascular morbidity and mortality, making it necessary to assess the prevalence of
thyroid dysfunction in PCOS patients; and what pathophysiological mechanisms of this, especially
related to sexual hormones, can favor the appearance and progression of that one.
It is a review of scientific articles, searched in the MEDLINE database, through PUBMED,
published in English between January 2010 and April 2018.
The prevalence of autoimmune thyroiditis (AIT) and higher levels of thyroid stimulating hormone
(TSH) is higher among carriers of PCOS. Patients with PCOS may develop thyroid disease
due to genetic predisposition; changes in metabolic profile such as insulin resistance and chronic
inflammation; and changes in hormonal profile due to an increase in estrogen/progesterone ratio
causing susceptibility to autoimmunity and development of antithyroid antibodies through an
increase of the inflammatory cytokines and decrease of inhibitory mechanisms on the immune
system.
Due to the strong association between PCOS and AIT, it is recommended to screen for antithyroid
antibodies and to monitor thyroid function in the follow-up of women with PCOS. Management
of the metabolic syndrome allows control of the chronic inflammation that may impact on thyroid
function. Treatment of oligoanovulatory cycles may reduce the risk of onset and/or progression of
AIT related to dysfunction on estrogen/progesterone ratio.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common endocrine diseases in women of reproductive
age, with a prevalence of 5-10% according to the Rotterdam criteria of 2003 [1]. Based on these criteria,
the diagnosis of PCOS is established from at least two of the following three discoveries: polycystic ovary
through pelvic ultrasound; hyperandrogenism (clinical and/or biochemical); and oligovulation and/or
anovulation [2], after excluding other differential diagnoses such as hyperandrogenic syndrome (congenital
adrenal hyperplasia, androgen secreting tumors, Cushing’s syndrome) and prolactinomas [2,3]. The
disorder is characterized by an endocrine and metabolic profile that contributes to the early pathogenesis
of atherosclerosis and an increase in cardiovascular risk, with dyslipidemia, metabolic syndrome, insulin
resistance and type 2 diabetes mellitus [4,5], with reproductive implications, being the most frequent cause
of female infertility due to anovulation being frequently associated with menstrual irregularities [6], besides
having psychological repercussions [7].
Thyroid dysfunctions are also among the most common endocrine diseases in the general population [5]. Hypothyroidism is a known cause of clinical profile with manifestations similar to those of PCOS [8]. Hypothyroidism can affect gonadal function and fertility [4], being able to increase ovarian sensitivity to the action of gonadotropins and cause hypertrophy of the ovaries and also the formation of multiple follicular cysts. In addition, it can cause menstrual irregularities and ovulatory dysfunction [6]. For this reason it is necessary to exclude the possibility of hypothyroidism before the diagnosis of PCOS [8].
Autoimmune thyroiditis (AIT), the main form of which is Hashimoto’s thyroiditis (HT), also called chronic lymphocytic thyroiditis, is the most frequent cause of autoimmune disease; and hypothyroidism [9], due to the destruction and fibrosis of parenchymal tissue [10]. AIT affects 5-20% of the young female population and is the main cause of hypothyroidism in women [10]. The gold standard for the diagnosis of HT is based on histological examination, with findings of fibrosis and lymphocytic infiltration, although currently such examination is not done routinely because it is invasive and due to availability and cost [9]. Thus, the diagnosis has been made from the detection of high levels of anti-thyroperoxidase antibody (anti-TPO) and/or anti-thyroglobulin antibody (anti-Tg) in the serum and a thyroid image characteristic of ultrasound - hypoechogenicity or heterogeneous echotexture [9]. Subclinical hypothyroidism (SCH) is defined as high levels of thyroid stimulating hormone (TSH) with normal levels of free thyroxine (FT4). However, the diagnosis of SCH is dependent on the definition of upper limit of TSH, which remains a topic of debate [1]. The prevalence of hypothyroidism considering TSH above 4.5mlU/L in women of reproductive age is about 4% and the percentage increases with advancing age. Therefore, thyroid dysfunctions among young women are more likely to be represented by normal hormonal function with findings suggestive of progressing autoimmune disease (high titers of thyroid antibodies and/or characteristic sonographic imaging) [9].
There is a growing body of evidence demonstrating that thyroid autoimmunity and thyroid hormone dysfunction are more common in women with PCOS than in non-PCOS patients [2,3,11,12,13], especially subclinical hypothyroidism (SCH) [11]. However, the mechanism of association is still uncertain [5]. Several similar factors, such as genetic susceptibility and inflammation/autoimmunity, contribute to the pathogenesis of PCOS and thyroid dysfunction, suggesting a potential pathophysiological link between the two diseases [12]. In addition, factors related to sex hormones could stimulate autoimmune response and, thus, favor the development of autoimmune disorders in women [6].
Considering the growing evidence of AIT and hypothyroidism in patients with PCOS, the present study aims to evaluate the prevalence of the association between thyroid disease and PCOS; and to review the possible pathophysiological factors involved, with emphasis on sex hormones.
Methods
This is a review of scientific articles, searched in the Medical Literature and Retrivial System on Line
database (MEDLINE), through PUBMED, using a search with the following descriptors: (PCOS [title]
OR polycystic ovary syndrome [title]) AND (thyroid [title] OR thyroiditis [title] OR autoimmune [title]
OR autoimmunity [title] OR hypothyrodism [title] OR TSH [title]). All included articles were published
in English language and between January 2010 and April 2018, the complete text of which was available.
We excluded studies that did not meet the inclusion criteria previously mentioned.
Thyroid Disease in Patients with PCOS
Recent studies have demonstrated an association between polycystic ovary syndrome (PCOS) and
hypothyroidism [5]. Gaberšček et al. [6] in their review study cite that the first systematic prospective study
[14] addressing the prevalence of AIT in women with PCOS included 168 healthy controls and 175 women
with PCOS, with a mean of 28 years; and that in this study we found a prevalence of 26.9% of positivity for
anti-TPO and/or anti-Tg antibodies in those with PCOS against 8.3% of positivity in the control group.
Since TSH levels were significantly higher among those with PCOS, the hypoechoic ultrasound pattern
characteristic of HT was present in 42.3% of these patients versus 6.5% in women without PCOS [6]. In this study, 36 of the 175 patients with PCOS had established HT (20.6%), while only 11 of the 168 women
in the control group had HT (6.5%). Thus, the prevalence of autoimmune thyroid disease, specifically HT,
was about three times higher in women with PCOS when compared to controls [6].
An Italian study developed by Garelli et al. [13] to evaluate the prevalence of autoimmune thyroid disease and other non-organ autoimmune pathologies in 113 women with PCOS described a higher prevalence of AIT in these patients compared to 100 patients in the group control (27% versus 8%, p < 0.001). In the mentioned study, AIT was present in 30/113 patients with PCOS. Of these 30 patients, the typical ultrasound pattern of thyroiditis was observed in 28 patients; the presence of two antithyroid antibodies (anti-TPO and anti-Tg) was detected in 16 patients; in 10 patients there was only anti-TPO; and in 2 patients they had only anti-Tg positive antibody. All 30 patients with associated PCOS and AIT had normal levels of free T4 and only one had subclinical hypothyroidism [13].
Muscogiuri et al. [15] performed a study with 50 women with PCOS to investigate the association of AIT and levels of 25 (OH) vitamin D. In this study AIT was diagnosed when anti-TPO antibodies exceeding 80U/ml and/or anti-Tg antibodies levels exceeding 70U/ml. It was observed a prevalence of 24% AIT in the patients studied [15]. Furthermore, 25 (OH) vitamin D levels were significantly lower in AIT patients when compared with patients without AIT (32.0 ± 22.6 versus 49.6 ± 19.9 nmol/L; p = 0.02) [15].
In the original study by Benetti-Pinto et al. [16], where 168 Brazilian women with PCOS were recruited, with an age average of 24 years, prevalence of subclinical hypothyroidism (SCH) was observed in 11.3% of these patients. Since SCH is a condition found in approximately 4% to 10% of the general population, being about 2% in young women between 12 and 39 years old, it can be said that the prevalence found in this study was higher than expected [16].
Duran et al. [17], on the other hand, did not find evidence of association of AIT and PCOS in their study group composed of 73 women with PCOS and 60 age-adjusted controls. If positivity of antithyroid autoantibodies and/or thyroid heterogeneity were considered as criteria for AIT, the frequency of autoimmune dysfunction in those with PCOS was 32.5% versus 23.3% in those in the control group, with no statistically significant difference (p = 0.394). In their work, the authors suggest studies with larger samples and with objective and standardized modalities of laboratory and image [17].
In a study conducted by Arduc et al. [12] to investigate the relationship between PCOS and HT and the possible effects of endocrine-metabolic factors of PCOS on thyroid autoimmunity, 86 women with PCOS and 60 women without PCOS who body mass index (BMI). Women with PCOS had significantly higher levels of anti-TPO and anti-Tg when compared to the control group (p = 0.017 and p = 0.014, respectively). Anti-TPO levels were positive in 23 (26.7%) of the 86 patients with PCOS compared to only 4 (6.6%) positive in the control group (p = 0.002). Positivity for anti-Tg was found in 14 (16.2%) of the PCOS group versus 3 (5%) in the control group (p = 0.039). HT was detected in 19 (22.1%) of those with PCOS and in only 3 (5%) of the control group (p = 0.004). Finally, TSH levels were higher in the PCOS group than in the control group (p = 0.037) [12].
Kachuei et al. [10] in a case-control study with 78 patients with PCOS and 350 in the control group, observed significantly higher anti-TPO levels among those with PCOS (p = 0.040). However, the anti- TPO positivity did not differ between the case and control groups, with respectively 30.6% and 27.8% (p = 0.730). Anti-Tg levels were not statistically significant (p = 0.467), and although the anti-Tg positivity was higher among those with PCOS than among the control group (36.4% versus 30%), this finding was not significant (p = 0.467). TSH levels also did not differ significantly between patients with PCOS and women in the control group (2.77 ± 3.8IU/mL versus 2.29 ± 1.5IU/mL, p = 0.09). The authors suggest further studies and that larger samples may result in more statistically significant results [10].
In the original study by Yu and Wang [3], 100 women diagnosed with PCOS and 100 women in the control group, matched for age and body mass index (BMI), were evaluated in order to analyze thyroid dysfunctions in these groups of patients. In this study, it was observed that PCOS is associated with a high incidence of AIT and subclinical hypothyroidism (SCH) [3]. Significant levels of TSH (p < 0.001) and anti-TPO (p < 0.01) and lower triiodothyronine, also known as T3, (p < 0.001) were found in the group of women with PCOS compared to the control group. T4 levels were slightly lower in women with PCOS, but without statistical significance. In addition, in this study the ultrasound finding of characteristic hypoechogenicity was significantly higher in women with PCOS compared to controls (34% versus 7%, p < 0.01). AIT, whose diagnosis in the aforementioned study was based on the presence of anti-TPO antibody associated with hypoechogenicity on ultrasound examination, was more prevalent and significantly in the PCOS group in relation to control (25% versus 2%, p < 0.001) [3]. As for thyroid hormone dysfunctions, women with PCOS also had a significantly higher prevalence. Overt hypothyroidism was present in 3% of the women with PCOS and in no individual in the control group (p < 0.01); and subclinical hypothyroidism was observed in 27% of the PCOS group versus 8% in the control group (p = 0.0002). The state of euthyroidism was markedly higher in those in the control group than in women with PCOS (91% versus 68%, p < 0.0001). There was no statistical difference regarding hyperthyroidism [3].
From the majority of studies, it can be concluded that the association between PCOS and AIT, specifically HT, is very common. In addition, elevated TSH levels are common findings among patients with PCOS. AIT is more prevalent in women with PCOS than non-carriers; as well as TSH levels are also higher in PCOS patients compared to their respective controls. The main findings of these studies are reported in table 1. The next section will discuss the possible etiological and pathogenic factors for the occurrence of these phenomena.
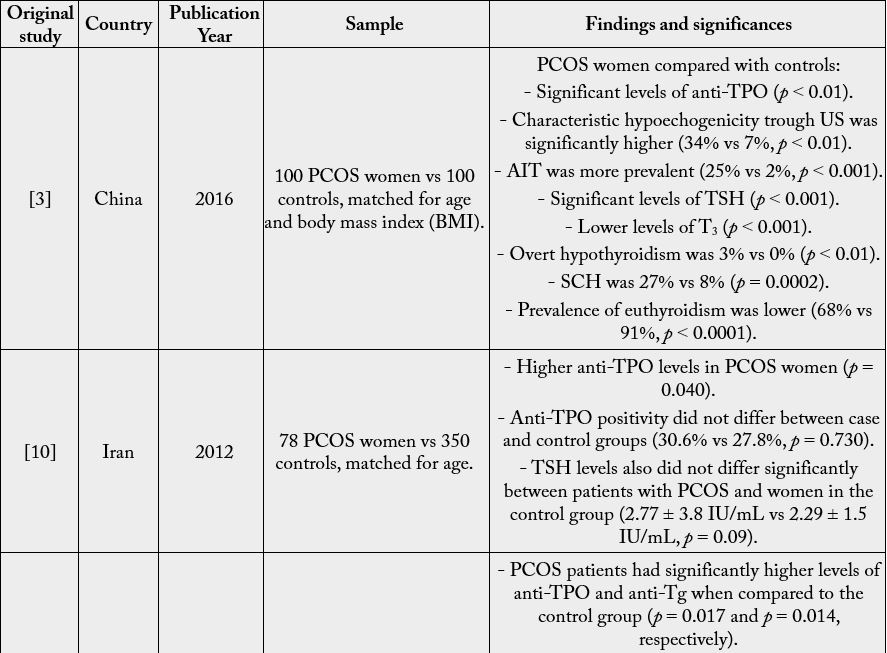
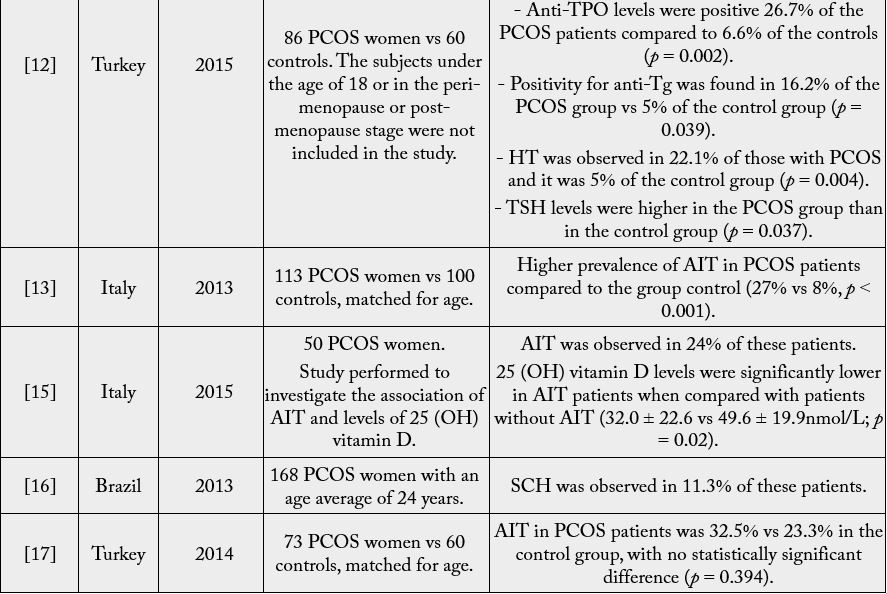
PCOS: polycystic ovary syndrome. vs: versus. Anti-TPO: anti-thyroid peroxidase. AIT: autoimmune thyroiditis. TSH: thyroid stimulating hormone. US: ultrasound. T3: triiodothyronine. SCH: Subclinical hypothyroidism. Anti- Tg: Anti-thyroglobulin. HT: Hashimoto’s thyroiditis.
Pathogenesis
The exact pathogenic mechanism of polycystic ovary syndrome (PCOS) is still a topic of debate, being
considered a heterogeneous disease with genetic and environmental components [15]. Endocrine-metabolic
aspects play an important role, such as gonadotropin concentrations [7], of sex hormones [12], and
hyperinsulinemia [6]. In addition, the presence of high levels of anti-ovarian antibodies in some women with
PCOS suggests a related autoimmune factor [12], although the role of autoimmunity in the pathophysiology
of PCOS remains controversial [18]. The etiology of AIT is not fully elucidated and it is considered that the
interaction between genetic susceptibility and environmental factors acts as a trigger for the installation and
progression of thyroid autoimmunity [12].
In HT is observed a strong genetic predisposition through family and twins. Kowalczyk et al. [9], in a review, mention a study in which children and siblings of patients with HT, the risk of developing HT is increased by 32 times and 21 times, respectively, with predominance in women. Gaberšček et al. [6] mention that 73% of the susceptibility for the development of thyroid autoantibodies can be attributable to genetic factors based on study carried out with Danish twins. Several genes are known to be associated with the disease onset, progression and severity: human leukocyte antigen (HLA-DR); CD40; cytotoxic T-lymphocyteassociated protein 4 (CTLA4); protein tyrosine phosphatase 22 (PTPN22); interleukin 2 receptor, alpha (IL2RA); vitamin D receptor (VDR); and, the only thyroid specific gene associated with HT, the gene for thyroglobulin (Tg) [6].
In PCOS, familial aggregation is well established [6,9]. Presence of PCOS features has been documented more frequently in first-degree relatives of women with the disease [6,9]. It has been documented several genes suspected of influencing PCOS development, but the results of research on most of them are inconclusive. Candidate genes are: fibrilin 3 (FBN3); insulin; insulin receptor; insulin receptor substrate 1; transcription factor 7-like 2; calpain 10; fat and obesity associated gene (FTO); sex-hormone-binding globulin (SHBG); and VDR [6]. Lately, in genome-wide association studies (GWAS), was identified the DENNDD1A gene, coding a protein participating in endosomal membrane transport, as a susceptibility gene for PCOS in an Asian population [9].
The pathogenesis of the association between PCOS and AIT is unclear [13] and data regarding etiology, pathogenesis and clinical implications are still scarce [6]. Considering that both PCOS and AIT present familial aggregation and genetic predisposition, the association between these diseases could be genetically determined. Several genes for susceptibility have been proposed as involved in the etiology of both diseases, but to date there is no well-established common genetic basis [6,9,12]. Three genetic polymorphisms could be involved in the pathophysiology of PCOS as well as of HT: FBN3, GnRHR and CYP1B1 [6].
Polymorphisms of FBN3 have influence on activity and levels of transforming growth factor beta (TGF beta) via fibrilins (FBNs). TGF beta acts as a key regulator of immune tolerance by stimulating suppressive Tregs and inhibiting T cell differentiation [6,9]. In hypothyroid HT lower serum levels of TGF beta when compared with healthy controls has been evidenced [6]. Moreover women with PCOS carrying allele 8 of D19S884 in the FBN3 gene have lower levels of TGF beta when compared with allele 8-negative PCOS patients, and, thus, theoretically they are more prone to develop HT. In relation to this, further investigations need to be performed [6,9].
A relationship between the 3’-UTR variant rs1038426 of gonadotropin-releasing hormone receptor (GnRHR) and insulin metabolism in PCOS women as well as an association with thyroid function in those patients is possible. A relation with serum levels of TSH and that genetic variant has been shown [6,9].
The CYP1B1 gene is associated with PCOS and encodes an enzyme that oxidizes estradiol to 4-hydroxiestradiol. Polymorphism CYP1B1 L432V (rs1056836) was related with serum levels of thyroid hormones [6,9].
The potential pathogenic link between PCOS and AIT may involve inflammatory mechanisms [19]. About 50 to 70% of all women with PCOS have some degree of insulin resistance (IR) [20] and this is an important risk factor for the development of type 2 diabetes mellitus. It has been shown that patients with PCOS have high levels of cytokines such as tumor necrosis factor alpha (TNF alpha) and interleukin 6 (IL-6), regardless of the presence of obesity [12,13,19], with PCOS being characterized as a low-grade inflammatory condition [13]. IL-6 is a potential mediator of autoimmunity by T3 lymphocytes. Through IR and an inflammatory environment there may be a reduction in deiodinase-2 activity and thus cause a relative decrease in T3 and an increase in TSH [3].
Probable etiopathogenic mechanisms for the onset of subclinical hypothyroidism (SCH) in patients with PCOS may be associated with elevated BMI and obesity [3]. Approximately 50% of PCOS patients are overweight or obese and many show increased abdominal circumference [21]. It is known that obesity increases the susceptibility to the installation of inflammatory and autoimmune diseases [21]. Adipose tissue is one of the most important sources of proinflammatory cytokines, as insulin resistance (IR) and hyperinsulinemia are related to the elevation of these cytokines. Thus, in synergism, insulin resistance and obesity in these patients cause an increase in adipocytokines and other inflammatory markers [19]. Another aspect to be considered is that obesity leads to increased levels of leptin, which would stimulate the hypothalamus to increase the secretion of thyrotropin-releasing hormone (TRH). This pathway, together with other mechanisms, could explain the higher incidence of SCH in women with PCOS [3]. In view of the above, the chronic inflammatory environment present in PCOS carriers may be a potential trigger for thyroid autoimmunity.
Vitamin D deficiency has been linked to the pathogenesis of autoimmune thyroid disease and PCOS [9]. It has been shown to have a positive impact on the immune system and is believed to be able to prevent autoimmune diseases. Therefore, vitamin D deficiency may be involved in an increased risk of autoimmunity [9]. Vitamin D exerts its effects through binding at its receptor, which is expressed in a wide variety of tissues, such as lymphocytes, monocytes and dendritic cell [6]. There is an increased prevalence of vitamin D deficiency [25 (OH) vitamin D] in patients with AIT [15]. There is evidence that calcium and vitamin D supplementation may promote the reduction of inflammatory markers and oxidative stress [9]; and that in women with PCOS this supplementation may improve menstrual frequency and metabolic disorders [6], since low 25 (OH) vitamin D level has a negative impact on metabolism, worsening insulin resistance, hyperinsulinemia and hyperandrogenism [9].
There is evidence of high prevalence of PCOS phenotype in adolescents with HT with normal thyroid hormone function. This suggests that the autoimmunity state itself may play an etiopathogenic role in the PCOS phenotype, regardless of the presence of hypothyroidism [12,19]. Histological findings of autoimmune oophoritis have been observed in some patients with PCOS [12,13]. In addition, some well-known autoimmunity markers such as anti-histone antibody, anti-DNA antibody, antinuclear antibody and smooth muscle autoantibody have been reported with high titers in some patients with PCOS [12]. High prevalence of lupus has been observed in women with PCOS. These findings suggest possible autoimmune mechanisms in the pathogenesis of PCOS [10,12,18]. Reinforcing the hypothesis of autoimmune etiopathogenesis in the association between PCOS and AIT, several original studies have demonstrated high levels of positivity of antithyroid autoantibodies such as anti-TPO and anti-Tg in groups of women with PCOS in relation to their respective controls [3,6,12,13,15]. Although the link between PCOS and autoimmune diseases is the subject of many studies, the results are conflicting and the exact role of autoimmunity in this association remains controversial [13,18].
It is known that the thymus plays an important role in the modulation of the immune system [6,9]. The maintenance of self-tolerance and consequently the prevention of autoimmunity is done through two mechanisms. The first is central immune tolerance with the deletion of autoreactive T cells by the thymus during fetal life. The second mechanism is peripheral immune tolerance promoted mainly by regulatory T cells (Tregs), derived from the thymus and from inactive lymphocytes (“naive cells”), which has a suppressive effect on the immune system and thus prevent excessive immune response [6,9]. Through animal models, there is evidence of immunological dysregulation induced by elevated levels of estrogen during the prenatal period, specifically before 10 days of gestation, when the thymus is in the final stage of maturation. It is believed that such elevated levels of estrogen can affect the maturation of the thymus and lead to the cessation of Tregs production, which may lead to the formation of multiple ovarian cysts, which supports the hypothesis of the autoimmune etiology of PCOS [6]. In addition to estrogens, adrenal steroids such as corticosterone have already been identified in animal models as causing a decrease in the number of thymocytes and the weight of the thymus; and also as factors for the development of ovarian cysts and anovulation [9]. Thus, dysregulations in the fetal thymus could be involved in immunological intolerance and onset of PCOS and AIT in predisposed individuals [6].
The role of sex steroid hormones in the pathogenesis of autoimmune diseases is plausible, since women are significantly more affected by these pathologies than men. Five percent of the world population has autoimmune disease, with 78% being women [6]. The prevalence of HT is 5 to 10 times higher in women than in men, reinforcing the hypothesis that factors involving sex hormones may be involved in the genesis [12]. In addition, the condition of excess estrogen has been related to different autoimmune diseases, considering the higher incidence of these pathologies in women of reproductive age, as well as has been observed in hypogonadal men, who have relatively high levels of estrogens [2]. It is known that abnormalities in sex hormone levels and balance between them are strongly associated with PCOS [12], with a relative predominance of estrogen over progesterone, and this may have an influence on the etiopathogenesis of autoimmune diseases, including HT [22].
The Role of Sexual Hormones
Theoretically, hormones such as follicle stimulating hormone (FSH), luteinizing hormone (LH) and estrogen
have a role in the pathogenesis of thyroid disease in women, which is supported by the finding that thyroid
diseases are more common in females [5]. Sexual steroids play an important role in the regulation of the
immune system [12,19] and estrogen is capable of activating the inflammatory and immune response [19].
Estrogen can stimulate the immune system by increasing the secretion of interleukin 1 (IL-1) in monocytes,
IL-6 in lymphocytes, interferon gamma and tumor necrosis factor alpha (TNF-alpha) [12]. In addition, it
has been reported that estrogens decrease the activity of suppressor T cells, increase B-cell activity and direct
the Th2 immune response and antibody formation [6]. Thus, female susceptibility to autoimmune diseases
is probably increased by the stimulatory action of estrogens in the immune system.
On the other hand, supraphysiological doses of estrogens, such as during pregnancy and in use of oral contraceptives, appear to cause immunosuppression [9]. Clinical manifestations of rheumatoid arthritis and multiple sclerosis tend to alleviate during pregnancy, especially in the third trimester, coinciding with higher levels of estrogen and progesterone [9].
In turn, progesterone has an inhibitory effect on the immune system by blocking lymphocyte proliferation, reducing the oxidative burst of monocytes, reducing IL-6 secretion by monocytes, and altering cytokine secretion in cell clones T to favor the production of IL-10 (an inhibitory cytokine) [12]. The decrease in the peripheral production of antibodies and the proliferation of macrophages can also be attributed to the progestagenic action [6,9]. It is known that autoimmune diseases in women predominantly exacerbate during the menarche and early menopause that are characterized by insufficient progesterone production [12].
Androgens are related to the suppression of the immune response [6,12,23] and are involved in the reduction of many elements of the immune system; in the suppressor T cell activity improvement; in improving the Th1 response; and activation of CD8+ T lymphocytes [6]. Nevertheless, androgen levels in women with PCOS, although increased, are still insufficient to counteract the stimulatory mechanisms of high levels of estrogen and to suppress an excessive immune response [6,12,23].
Due to menstrual irregularities and numerous oligoanovulatory cycles, patients with PCOS have low levels of progesterone and normal or increased levels of estrogen [12]. In the study by Arduc et al. [12], where the association between the positivity of thyroid autoantibodies and sex hormones in patients with PCOS was evaluated, a positive correlation was observed between anti-TPO levels and estrogen levels and estrogen/ progesterone ratio in these patients; no correlation was observed between the presence of antithyroid antibodies and androgens.
Therefore, it is believed that in women with PCOS, the increased estrogen/progesterone ratio for long periods of oligoanovulation and luteal insufficiency may be involved in hyperstimulation of the immune system and, therefore, in the frequent occurrence of AIT in these patients [2,6,12,15,19,23]. Thus, treatment of oligoanovulatory cycles and restoration of normal menstrual cycles may reduce the risk of onset and/or progression of AIT in patients with PCOS [12]. The possible mechanisms related to sexual hormones in the pathophysiology of the association between PCOS and AIT are summarized in table 2.
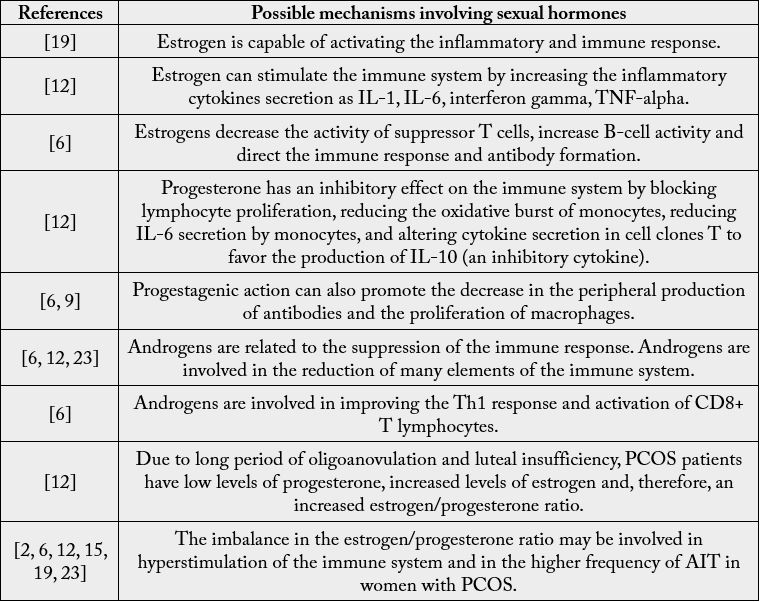
PCOS: polycystic ovary syndrome. AIT: autoimmune thyroiditis. IL-1: interleukin 1. IL-6: interleukin 6. TNF-alpha: tumor necrosis factor alpha. IL-10: interleukin 10. Th1: T-helper 1.
Conclusions
The prevalence of AIT, specifically HT, is significantly higher among patients with PCOS than among noncarriers.
Due to this strong association, it is recommended to perform screening for antithyroid antibodies
and monitoring of thyroid function in the follow-up of PCOS patients. In these patients, the management
of the metabolic syndrome allows the control of the chronic inflammatory state that may impact the thyroid
function. Treatment of oligoanovulatory cycles may reduce the risk of AIT onset and/or progression by
preventing hormonal factors, especially the increased estrogen levels and high estrogen/progesterone ratio,
that through stimulation of the immune response would lead to thyroid autoimmunity. However, with
regard to the etiology, pathogenesis and clinical consequences of the association between PCOS and AIT,
further investigations need to be performed.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!