Biography
Interests
Chukwurah Ejike Felix1, Obeagu Emmanuel Ifeanyi2* & Chukwu Ngozi Bethel3
1Department of Haematology and Immunology, Faculty of Clinical Medicine, Ebonyi State University, Abakaliki,
Nigeria
2Department of Medical Laboratory Science, Faculty of Health Sciences, Imo State University, Owerri, Nigeria
3Department of Medical Laboratory Science, Faculty of Health Sciences, Ebonyi State University, Abakaliki, Nigeria
*Correspondence to: Obeagu Emmanuel Ifeanyi, Department of Medical Laboratory Science, Faculty of Health Sciences, Imo State University, Owerri, Nigeria.
Copyright © 2019 Obeagu Emmanuel Ifeanyi, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study aimed to evaluate the prothrombin Time and Activated partial thrombin Time (APTT) of Albino rats, fed with varied concentrations of used engine oil. A total of twenty Wister Albino rats aged 10 (ten) weeks and weighing 97-99kg were used. The rats were divided into five groups containing 4 rats each. Orally administered doses were 0.2ml, 0.4ml, 0.6ml. 1ml and in feed are 1ml. 2ml, 3ml and 4ml of used engine oil corresponding to groups B, C, D and E, respectively for 28 days while group A (control) received uncontaminated food and water. The sample collection was through retro-orbital plexus, the sample were collected into a plain plastic tube containing trisodim citrate citrate anticoagulant in the ratio 9:1 (2ml of blood in 200ul trisodium citrate) and calcium chloride. The Prothrombin time samples were analysed by platelet poor plasma concentrate method and APTT with Kaolin reagent. The Analysis of variance (Anova) expressed in mean+ standard deviation showed dose dependent significant increase in the prothrombin Time 0.2ml dose (15.00±11.63), 0.4ml (33.00±3.06). 0.6ml (34.00±3.50) and APTT 0.2ml (39.00±2.63). 0.4ml (105.00±5.03), 0.6ml (108.00±2.38) compare to the control group. Anova table shows significant difference and decrease in total body weight of treated groups were observed then compared to the control group (P<0.05). The available information shows that used engine oil prolongs prothrombin time, inhibiting clothing factor (antithrombotic). Proper disposal and recycling of used engine oil is strongly recommended.
Introduction
Engine oil is refined from crude oil or synthetic oil for lubricating of combustion engines. It also cleans, and
inhibits corrosion, improves sealing cools the engine by carrying heat away from removing parts. Engine oil is
composed of a engine base of oil (a complex mixture of hydrocarbons, 80-90% by volume) and performance
enhancing additives (10-20% by volume) [1,2].
The largest contributor to the oil spillage beside corrosion of pipes and tanks, is suggested to be the rupturing or leaking of production infrastructures that are described as “Very old and lack regular maintenance [3].
However, according to Dede et al. (2002) [4] cases of misuse of this substance by individuals have been reported, as it is known to be liberally used by some of the indigenes who believe that it can repel witches when applied either topically or given orally to affected individuals. While other countries such as Kenya, Tanzania. Zimbabwe. Ghana and Tunisia depends on crude oil for unorthodox treatment of ailments such as stomach ache. Crude oil is orally ingested for medicinal purposes [5].
Generally, various studies on crude oil have revealed that it has serious deleterious effects on soils (Jeroh et al., 2001). Aquatic life (Daka and Ekweozor. 2004) [6] and even organisms such as the macrobenthic invertebrates (Arimoro and Adamu, 2008). However, humans and other animals are also adversely affected. The constituents of crude oil can irritate the skin and mucous membrane on contact, irritant effects can range from slight reddening to burning, swelling (Oedema), pain and permanent skin damage.
Commonly reported effects of acute exposure to crude oil through inhalation or ingestion includes: Difficulty in breathing, headaches, nausea, confusion and other central nervous system effects (Akpofure et al., 2000). Chronic, exposure of animals to crude oil produces signs and symptoms of toxicity involving the central nervous system (Onuoha and Nwadukwe, 1990), the reproductive system [7] as well as genetoxic (Kalf et al, 1987). Aslani et al, (2000) [8] reported bloody stools, coughing, constipation, infertility and sudden death in female goats exposed to West Texas Intermediate crude oil. while studies conducted by Igwebuike et al. (2007) [9] revealed that exposure of male rats to Nigerian Qua-Iboe brent resulted in reduced packed cell volume, increased total leukocytes count and reduced Cauda epididymal sperm reserves.
Therefore, there is need to investigate the effect of contaminated food and water with used engine oil in relation to coagulation profile of Albino rat.
Coagulation protects animals from excessive loss of blood and fluids by sealing the sites of injury and restoring vascular integrity. The processes of clot formation and fibrinolysis involve highly complex and interdependent cellular vascular, enzymatic regulatory events [10].
The extensive quantitative differences in coagulation system among mammals species imply the need for species-specific studies of coagulation properties [11]. Blood coagulation has been most extensively studies in humans. Several studies have also been varied out on the hemostatic profile of domestic and common laboratory lack of species-specific laboratory reagents, these studies are usually carried out using the same diagnostics employed for measuring clotting factors in human plasma. Hussein et al, (2009), the coagulation profiles of desert mammals have received little or no attention. Therefore, there is need to investigate the coagulation profile of Albino rats given contaminated food and water with used engine oil.
Aim
To evaluate the Prothrombin time and Activated partial thrombin time in Albino rats fed with different concentration of used engine oil.
Objectives
1. To assay the prothrombin time on Albino rats fed with varied concentration of used engine oil.
2. To assay the Activated partial thrombin time of the rats.
3. To compare the results or relate the findings to different concentration of Used engine oil.
Materials and Methods
A total of 64 individuals fish and 5 selected species was collected. Table 1 shown the fish species with total
length and weight. All fish collected known to be herbivorous feeding habits.
The experimental animals were wistar strain albino rats of both sexes of (10) ten weeks of age, bred and
acquired from the Genetics and Animal breeding laboratory/house department of Anatomy, Ebonyi state
university.
The used engine oil was sourced from mechanic village, Abakaliki. Ebonyi State
- APTT Reagent
- 0.025M Calcium Chloride Reagent
- Thromboplastin reagents
- Laboratory centrifuge
- Water bath
The rats were carried, kept in a clean and well ventilated wire mesh cages in animal house under standard
conditions (temperature 25 - 29°c). The rats weight between 97-173kg and was divided into five (5) groups,
4 in each cage according to their weight were fed alibitum with chicken growers mash feed commercially
prepared and clean drinking water.
The animals were divided into five groups (A, B, C, D, E). They were allowed to acclimatized for two weeks before treatments commenced.
Group A rats were used as control while group B to E were administered with varied concentration of used engine oil (UEO) of 0.2ml, 0.4ml, 0.6ml and 1ml per litre of water respectively and 1.0ml, 2.0ml, 3.0ml and 4.0ml per kg of feed respectively.
Group B (low dose)
Four rats were in this group, each rat received a low dose of used engine oil orally (0.2ml) and 1.0ml in feed, for a period of four weeks. Group C (medium dose). There are four rats used, each received a medium dose of used engine oil orally (0.6ml) and 2.0ml in feed for a period of four weeks. Group D (High dose). There are four rats used, each received a high dose of used engine oil orally (0.6ml) and 3.0ml in feed for a period of four weeks. Group E (Higher dose). There are four rats were used in this group, each received a higher dose of used engine oil orally (1.0ml) and 4.0ml in feed for a period of four weeks. Group A (Control) In this group, four rats were used, each received uncontaminated feed and drinking water with used engine oil for a period of four weeks.
A Perkin Elmer FT-IR Spectrometer Spectrum Two Universal ATR was used to collect spectra from 4000cm−1 to 450cm−1 with a data interval of 1cm−1. Resolution had been set at 4cm−1. The ATR diamond crystal clean up with 70% ethanol and a background scan was performed between each sample. Each sample was compressed against the diamond to ensure good contact between sample and ATR crystal, as recommended by Perkin Elmer. Absorption bands identified by using a peak height algorithm within the Perkin Elmer software and compared to absorption bands of each polymer reported in the literature and obtained from in-house spectral library.
Identification of the Animals
For easy differentiation of each of the rats in the different cages, they were marked by tattooing at the tail
region, hands and legs with 2 different coloured markers.
Base line was collected after acclimatization and before introduction/administration of varied concentration
of used engine oil. After administration of the food and water contaminated with varied concentration of
used engine oil for 14 and 28 days to group B, C, D and E, while group A received normal water and feed,
the sample were collected into plain plastic tube containing trisodium citrate anticoagulant in the ratio 9: 1
(i.e 2ml of blood in 200ul trisodium citrate) and calcium chloride for coagulation assay. The samples were
assayed straight without being preserved in the refrigerator.
The laboratory procedures for administration and collection:
Route of administration is orally. Collection of blood is retro-orbital plexus procedure was utilized in the collection of the sample. The rat was picked and head was turned towards the left so that the right eye can be approached A capillary tube was gently and firmly inserted into the medial canthus of the right eye. The tube was rotated while inserting, so that it can cut the nerve plexus. It was slightly withdrawn, so that blood would come out. The capillary tube was held in same position till blood stopped. Gently, it was removed and rat released immediately.
All data obtained were analysed, result were expressed as mean ± standard deviation using using the statistical
package for social science (SPSS) version 12. Analysis of Variance (ANOVA) used to compare and
evaluate significant difference between means of control and the means of various treatment group. P values equal to or less than 0.05 were considered significant (Graphad Instat, 1998). All results were expressed as
mean ± standard deviation.
Sample Analysis
Method adopted was that of (Decie and Lawis, 2006) [12].
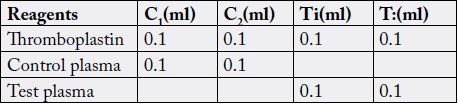
The test and control blood sample were centrifuged at 1000g for 15 minutes to obtain platelet poor plasma.
0.1lml thromboplastin was added into four glass tube (in duplicate of control and test tube i.e C1 and C2. TI
and T?) place in water bath at 37ºc for 7 minutes. 0.1ml of normal control plasma was then added to C1 and
C2, and 0.1ml test plasma to TI and T2 respectively, one at a time and then incubate for 3 minutes. Water
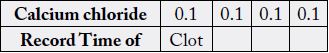
bath was open and 1.1ml of calcium chloride then added to each tube. The stop watch was immediately
switched on. The tubes were repeatedly tilted until clot forms. The time was recorded (Ochei and Kolhaktar,
2008) [13].
Method adopted was sited from (Decie and Lewis, 2006) [12].
The control test was done in duplicate with the patients plasma 0.2ml of a well mixed kaolin/platelet substitute
placed into a small glass tube labeled Ci,. Ti, ti Then 0.1ml of control and test plasma were added in
each tube, mixed and incubated at 37ºC for 2miuntes while tilting at intervals. After 2miuntes 0.1ml of Ca was introduced into the mixture and the stop watch on. The test tubes were held in water bath, while tilting
and looking for clot formation. The time for clot formation was then recorded for each mixture.
The APTT of the test was reported as an average of the duplicate test (Cheesbrough, 2004).
Result
Table 1 is mean and standard deviation values of prothrombin and Activated partial thrombin time before
administration of used engine oil (day 0).
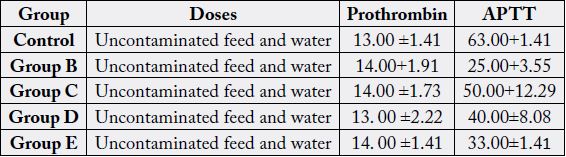
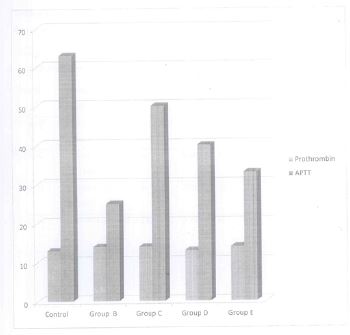
Table 2 the effect of used engine oil showed a significant (P0.05) increase in prothrombin (13.00+2.22) in the group administered with 0.2ml of used engine oil at the 14th day when compared to the control group. The group administered with 0.4ml of used engine showed a reduction in prothrombin (9.00+1.53) at the 14th day when compared to the control (12.00+1.41). A decrease in the prothrombin value was also recorded among the groups administered with 0.6ml and 1ml of the used engine oil.
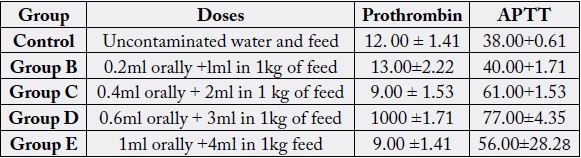
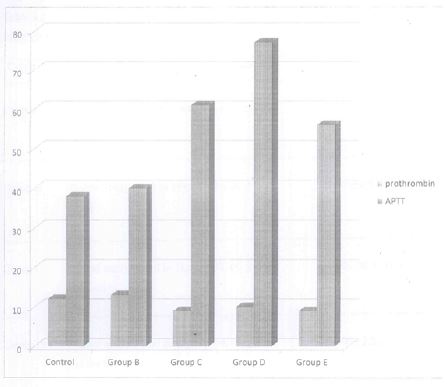
The result also recorded a significant (P<0.05) dose increase in APTT and a marginal decrease at a dose of 1ml (56.00+28.28) was obtained on the 141’1 day when compared with other doses, 0.2ml (40.00±1.71), 0.4ml (61.00±1.53) and 0.6ml (77.00±4.35). There is increase when compared with the control (38.00±10.61).
Table 3 showed a reduction in prothrombin (34.00±1.41) and APTT (73.00±10.61) at a high dose of 1ml on the 28th day when compared to the control groups (13.00+1.41 and 54.00+2.12) respectively. The results of other groups revealed a dose dependent increase in prothrombin and APTT at a dose of 0.2ml (15.00±1.63), 0.4ml (33.00±3.06) and 0.6ml (34.00±.1.41), with the same linear increased observed in APTT.
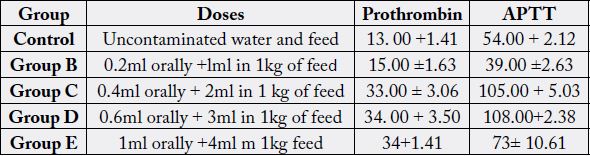
Discussion
The administration of used engine oil contaminated feed and water on the rats manifest in various forms
including changes in prothrombin time, activated partial thrombin time and body weight.
Previous researches revealed that some individuals may experience adverse effects when exposed to used engine oil due genetic polymorphism [14]. Increased risk of liver changes leading to prolonged coagulation and malfunctioning, squamous cell carcinoma in lungs, harmful effects on the kidney, heart and nervous system has been reported in a multi-site, case contain study [15]. Studies have implicated free radicals from exogenous and indigenous sources, is the etiology of a lot of degenerative diseases [16].
In this study the significantly increased of prothrombin time and Activated partial thrombin time in table 2 and 3 may have resulted from hepatic injury from oxidative stress from crude petroleum oil exposure. The hepatic injury inhibits the vitamin K-dependent clotting factors, which is assayed by Activated partial thrombin time. The activities of liver enzymes is reduced. Such as Aspartate Transaminase (AST), Alkaline Transaminase (ALT) etc.
Many other studies have identified that petroleum products, even used engine oil. Other agents with therapeutic usefulness have been recognized to be capable of altering physiologic processes that may culminate in abnormality of body or micronutrient [17].
Both rats and man have been reported to have similar level of toxicity/susceptibility to many drugs.
There is observed increase in prothrombin (13.00+2.22) in the group administered with 0.2ml orally and 1ml in 1kg of feed at the 14th day of table 4 when compared with control.
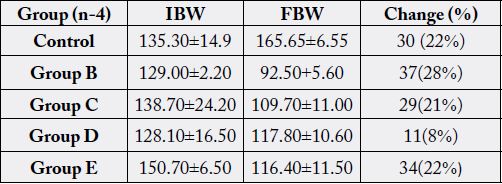
KEY IBW FBW
Initial body weight
Final body weight
The group administered 0.4ml, 0.6ml, 1ml orally and 2ml. 3ml. and 4ml in each kg of feed respectively. The decreased prothrombin time results is significant (P>0.05) statistically. After 28 day the prothrombin time increased significantly (P<0.05) (15.00±1.63. 33.00±3.06, 34.00±50, 34±1.4). for the respective dose of used engine oil administered. The result revealed dose dependent since Prothrombin Time is a screening test for extrinsic system (factor VII and detect factor V, X, prothrombin and fibrinogen) (Cheesbrough, 2004) this result agrees with the reported findings of [17].
Activated partial thrombin time (APTT) is qualitative measurement of factors involved in the intrinsic pathway.
In Table 2, after 14 days the result showed a significant (P<0.05) dose increase 0.2ml,0.4ml, 0.6ml (40.00±1.71. 61.00±1.53, 77.00±4.35) and a marginal decrease at a dose of 1ml (56.00±28.28) was obtained on 14th day when compared to control (38.00±10.61). The result showed a dose dependent of APTT at a dose of 0.2ml (15.00±1.63), 0.4ml (33.00+3.06) and 0.6ml (34.00±11.41) with the same linear decrease observed in APTT.
This result reflects increase in APTT, the clotting factors involved in intrinsic System which correlates with the report of Ritchie et al., 2010 [18-43].
Conclusion
The result of this investigation indicate that ingestion of used engine oil either orally, inhalation, , ingestion
affect coagulation. Or inhibit clotting factors, thereby prolonging clotting. The effect of used engine oil is dosage dependent and duration exposure. A short period of 14 days as seen in prothrombin results showed
a marked decrease but after 28 days exposure/duration, there is a significant increase. This shows that used
engine oil is toxic and affects the haemostatic mechanism and can cause bleeding disorders, since it increases
clotting time.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!