Biography
Interests
Khalid Aljabri, S.*, M.D., F.R.C.P.C., F.A.C.P. & Samia Bokhari, A., M.D., S.B.E.M.
Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia
*Correspondence to: Dr. Khalid Aljabri, S., Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia.
Copyright © 2019 Dr. Khalid Aljabri, S., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Growth hormone deficiency (GHD) is one of the most important endocrine and treatable causes
of short stature. Therefore, the objective of this report is to assess retrospectively the prevalence of
GHD in a cohort of Saudi population.
Retrospectively, a total of 47 patients were included for analysis from January 2015 to December
2018 at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia. There were 39 males (83.0%)
and 8 females (17.0%). For insulin-like growth factor (IGF-1) and Insulin-like Growth Factorbinding
Protein-3 (IGFBP-3), laboratory reference ranges were based on age and sex. This reference
range was used to categorize patients as either ‘‘IGF-1 Deficient’’ or ‘‘IGF-1 Normal. The insulin
tolerance test (ITT) provocative tests was used for diagnosing GHD. The ITT was performed
after an initial baseline blood draw. Regular insulin at a dose of 0.1 unit per body weight (Kg), was
administered intravenously, and blood draws occurred at 0 (baseline), 30, 60, 90, and 120 minutes for GH. Patients with a peak GH of ≤5.0ng/dl were considered to be GHD and patients with a
peak GH of ≥ 5.1ng/dl were considered not GHD (nGHD). Within the GHD patients, peak GH
of ≤ 3.0ng/dl were considered to be severely GHD (sGHD), and patients with a peak GH of 3.1-
5.0ng/dl were considered moderately GHD (mGHD).
We included 47 patients for analysis. Mean age was 14.6 ± 1.6 years. Mean IGF-1 and IGFBP-3
concentrations were 145.8 ± 70.1mcg/L and 3709.9 ± 1009.6mcg/L respectively. Results from the
ITT indicated that 27 (57.4%) had GHD. Age was not significantly different between GHD and
non-GHD (14.5 ± 1.7 years vs. 14.8 ± 1.6 years, p=0.6). There was non-significantly more males
than females in the GHD patients (59% vs, 50%, P=0.7). Mean IGF-1 and IGFBP-3 were not
significantly different (145.9 ± 71.6mcg/L vs. 145.7 ± 69.8mcg/L, p=0.9) and (3608.0mcg/L ±
1141.9 vs. 3816.8 ± 867.0mcg/L, p=0.5) respectively. IGF-1 and IGFBP-3 concentrations below
the reference ranges for age and gender were non-significantly higher in patients with GHD
compared to non-GHD, (53.8% vs, 46.2%, P=0.8) and (66.7% vs, 33.3%, P=0.7) respectively. The
mean peak for GH was significantly lower in patients with GHD (2.2 ± 1.3 1ng/dl vs. 9.9 ± 1.6ng/
dl, p<0.0001). GHD, ranging from sGHD to mGHD was found in 20 (42.6%) and 7 (14.9%)
respectively.
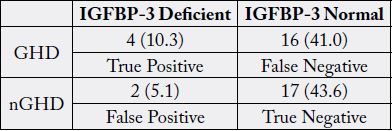
To address the accuracy and sensitivity of IGF-1 reference ranges in screening patients with GHD, IGF-1 concentrations were compared with ITT. 26.1% of patients whose IGF-1 concentrations fell within the normal reference range for their age and sex were GHD, indicating that if IGF-1 levels were relied on in isolation, a false negative result would have been obtained. The sensitivity and the specificity of IGF-1 for diagnosis of GHD were 54% and 40% respectively. Moreover, 41% of patients whose IGFBP-3 concentrations fell within the normal reference range for their age and sex were GHD, indicating that if IGFBP-3 concentrations were relied on in isolation, a false negative result would have been obtained. The sensitivity and the specificity of IGFBP-3 for diagnosis of GHD were 20% and 90% respectively.
We conclude that despite the limitations of this hospital-based retrospective study, GHD is highly
prevalent in cohort of Saudis investigated for short stature. This observation remains to be validated
by population-based studies.
Introduction
Growth hormone deficiency (GHD) is one of the most important endocrine and treatable causes of short
stature. The interest in the epidemiology of GHD derives from the increasing focus on patients with GHD
during the last decades. This interest was spurred on by the finding of positive changes in body composition
in patients with GHD being treated with growth hormone (GH) [1-4].
There are few data on incidence rates of GHD. Childhood onset GHD has been estimated to occur in 1 per 30 000 people per year [5]. In adult onset GHD, an annual incidence of 1.2 per 100 000 adults has been estimated [6]. To our knowledge, there have been no nationwide studies using uniform diagnostic and classification criteria for all citizens. The insulin tolerance test (ITT) is considered the ‘‘gold standard’’ in provocative tests for diagnosing GHD [5,6].
In a developing nation like Saudi Arabia, perception of height as a marker of general health is less pronounced as compared to weight, due to the social health system being more focused on common causes of malnutrition, rather than on normal growth and development. This factor might lead to underdiagnosis of pathologic causes of short stature and other conditions potentially associated with poor growth. Further, there is insufficient awareness of the need to measure height, height measurement techniques, and thresholds for referral among primary care physicians. It is often up to families to recognize that their child is not gaining proper height and seek advice from a primary care physician. In the majority of cases, the families wait until adolescence and late puberty, when chances to improve final height are limited. The prevalence of GHD in children with short stature ranges from 2.8% to 69% [7-10]. GHD is also estimated to be prevalent in more than 80% patients undergoing postneurosurgical procedure [11,12]. A study by Colaco et al., found that 31% of the children had familial GHD and about 17% of children had idiopathic GHD [13]. These studies are biased by patterns of referral to tertiary centres of care. Therefore, the objective of this report is to assess retrospectively the prevalence of GHD in a cohort of Saudi population.
Methods
Retrospectively, a total of 47 patients were included for analysis from January 2015 to December 2018 at
King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia. There were 39 males (83.0%) and 8 females
(17.0%). For insulin-like growth factor (IGF-1) and Insulin-like Growth Factor-binding Protein-3 (IGFBP-
3), laboratory reference ranges were based on age and sex. This reference range was used to categorize
patients as either ‘‘IGF-1 Deficient’’ or ‘‘IGF-1 Normal. After overnight fasting, the ITT was performed
after an initial baseline blood draw. Regular insulin at a dose of 0.1 unit per body weight (Kg), was administered
intravenously, and blood draws occurred at 0 (baseline), 30, 60, 90, and 120 min for GH. 5,6,14-17
Patients with a peak GH of ≤ 5.0ng/dl were considered to be GHD and patients with a peak GH of ≥5.1ng/
dl were considered not GHD (nGHD). Within the GHD patients, peak GH of ≤3.0ng/dl were considered
to be severely GHD (sGHD), and patients with a peak GH of 3.1–5.0ng/dl were considered moderately
GHD (mGHD). Blood was centrifuged, and serum was frozen with dry ice until analysis by an independent
laboratory (Unilabs Inc., Germany). GH levels were analyzed using an immunochromatographic membrane
assay.
Statistical Analysis
Continuous variables were described using means and Standard Deviations. Univariate analysis of baseline
demography both between groups, were accomplished using unpaired t-test and Chi square test were used
for categorical data comparison. P value <0.05 indicates statistical significance. The statistical analysis was
conducted with SPSS version 23.0 for Windows.
Results
We included 47 patients for analysis. Mean age was 14.6 ± 1.6 years (table 1). Results from the ITT indicated
that 27 (57.4%) had GHD (table 2). Age was not significantly different between GHD and non-GHD
(14.5 ± 1.7 vs. 14.8 ± 1.6, p=0.6). There was non-significantly more males than females in the GHD patients
(59% vs, 50%, P=0.7). Mean IGF-1 and IGFBP-3 concentrations were not significantly different (145.9
±71.6mcg/L vs. 145.7 ±69.8mcg/L, p=0.9) and (3608.0 ±1141.9mcg/L vs. 3816.8 ± 867.0mcg/L, p=0.5)
respectively. IGF-1 and IGFBP-3 concentrations below the reference ranges for age and gender were nonsignificantly
higher in patients with GHD compared to non-GHD, (53.8% vs, 46.2%, P=0.8) and (66.7%
vs, 33.3%, P=0.7) respectively. The mean nadir for GH was significantly lower in patients with GHD (2.2
±1.3ng/dl vs. 9.9 ±1.6ng/dl, p<0.0001). GHD, ranging from sGHD to mGHD was found in 20 (42.6%)
and 7 (14.9%) respectively (Table 3).
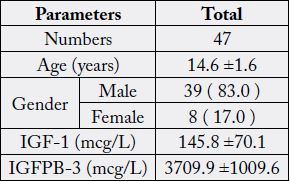
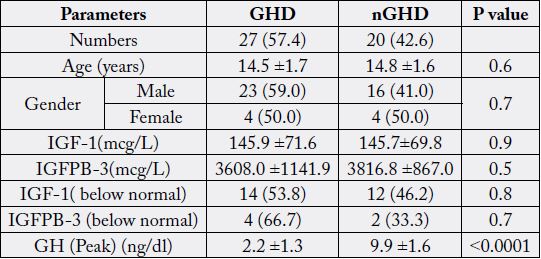
GH, Growth Hormone; IGF-1, Insulin-Like Growth Factor; IGFBP-3, Insulinlike Growth Factor-binding Protein-3
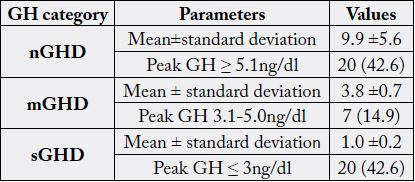
GH, Growth Hormone; mGHD, moderate Growth Hormone Deficiency; nGHD, non-Growth Hormone Deficiency; sGHD, severe Growth Hormone Deficiency
To address the accuracy and sensitivity of IGF-1 reference ranges in screening patients with GHD, IGF-1 levels were compared with ITT. According to standard reference ranges, based on age and sex, there were no patients with abnormally high concentrations of IGF-1. Patients were then further classified based on the results of the ITT, as either GHD or nGHD based on the results of the ITT. To be included in the GHD group, a patient needed to have a peak GH concentrations of ≤5ng/dl, which would include the sGHD and mGHD categories in this study. 26.1% of patients whose IGF-1 concentrations fell within the normal reference range for their age and sex were GHD, indicating that if IGF-1 levels were relied on in isolation, a false negative result would have been obtained (Table 4). The sensitivity and the specificity of IGF-1 for diagnosis of GHD were 54% and 40% respectively. Moreover, 41% of patients whose IGFBP-3 concentrations fell within the normal reference range for their age and sex were GHD, indicating that if IGFBP-3 concentrations were relied on in isolation, a false negative result would have been obtained (Table 5). The sensitivity and the specificity of IGFBP-3 for diagnosis of GHD were 20% and 90% respectively.
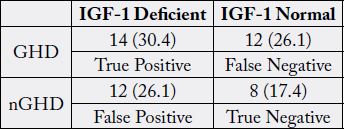
GHD, Moderate Growth Hormone Deficiency; nGHD, non-Growth Hormone Deficiency
Discussion
We found that 57.4% of patients in this study had a diagnosis of some level of GHD. Consensus guidelines
for GHD depends on peak GH concentrations after provocative testing [14-18]. This continuum was
demonstrated in the current study. The majority of the patients in this study fell within the sGHD category
(peak GH ≤3ng/dl), based on results of the ITT. Prevalence rates for GHD range from 6-33% [19-22]. The
higher prevalence of GHD in this study may be because of the use of the ITT to diagnose GHD and the
inclusion of patients who had mGHD.
This study provides further support that IGF-1 concentrations are not a good predictor of GHD. It has been reported that approximately 50% of patients with IGF-1 concentrations falling within the normal reference range are GHD [23]. In our study, 26.1% of patients whose IGF-1 concentrations was considered normal for their age and sex were confirmed to be GHD via the ITT, with a cutoff of peak GH ≤5ng /dl. If GHD were diagnosed solely on IGF-1 concentrations, a notable number of patients would have been missed and withheld from potential treatment.
In spite of these issues, literature suggests that IGF-I and IGFBP-3 are useful in the diagnosis of GHD. An obvious difficulty in determining the diagnostic performance of any test for GHD is the lack of a gold standard to determine true GHD; thus, reports regarding the sensitivity and specificity of IGF-I and IGFBP-3 are not consistent. In general, however, both IGF-I and IGFBP-3 are reported to have good specificity but relatively poor sensitivity for GHD which in concordance with our finding [24,25]. Irrespective of the published consensus guidelines that exist for the diagnosis of GHD, endocrinologists and other healthcare professionals often rely solely on IGF-1 concentrations to determine GHD, rather than evaluating GH with provocative testing. The results of our study support provocative testing for the diagnosis of GHD.
We aimed to identify the frequency of GHD. Furthermore, due to the retrospective nature of this study, the observed population reflects a selected yet comprehensive group of patients rather than the general population. Our study could be limited by the question of clustering of cases within the study region and the effect that might have on our estimates, in addition, the current study population may appear limited in size and therefore may underestimate the true frequency of GHD. In addition, the study shares the limitations of all retrospective studies.
Conclusion
We conclude that despite the limitations of this hospital-based retrospective study, GHD is highly prevalent
in cohort of Saudis investigated for short stature. This observation remains to be validated by populationbased
studies. In the absence of registry data, larger cooperative studies involving diverse population samples
from multiple centers could help to provide further information on the true frequency nationally.
Acknowledgment
The author would like to thank all colleagues from the Department of endocrinology for helping in data
collection.
Conflict of Interests
The authors declare no conflict of interests.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!