Biography
Interests
Denise, E. M.*, Akpan, E. N. & Anyadike, M. C.
Department of Botany and Ecological Studies, University of Uyo, Uyo, AkwaIbom State, Nigeria
*Correspondence to: Dr. Denise, E. M., Department of Botany and Ecological Studies, University of Uyo, Uyo, AkwaIbom State, Nigeria.
Copyright © 2019 Dr. Denise, E. M., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Phytotoxicity and the effects of water saturated fractions of Hexane on growth of microalgae was carried out using three species of fresh water microalgae namely Chlorella vulgaris (Chlorophyte), Nitzschia palea (Diatom) and Anabaena flosaquae (Cyanobacterium). The microalgae were grown separately in ratios 1:9 of 0%, 10%, 25%, 50%, 75% and 100% of water saturated fractions of Hexane. Algal growth was estimated using spectrophotometer using an absorbance of 745nm. Phytotoxicity was measured using percentage inhibition. C. vulgaris showed growth stimulation at 10% - 50% concentrations of the WSF while 75% and 100% concentrations were inhibitory. A. flosaquae showed growth stimulation in 10% - 75% of the WSF. N. palea showed growth stimulation at all concentrations (10% - 100%) of the WSF of the hydrocarbons. The inhibitory effects observed in this study at higher concentrations were more pronounced during the exponential growth phase. The order of order of growth stimulation or response of microalgae is N. palea> A. flosaquae> C. vulgaris. The results obtained showed mineralization pattern of the hydrocarbons by the different species of microalgae and thus could be an addition to existing list of indicators microalgae of crude oil hydrocarbon polluted environment and microorganism with hydrocarbon bioremediation potentials. This results obtained from this study could be an addition to existing data on the subject and hence useful in environmental management.
Introduction
Crude oil is a homogeneous substance comprising different complex mixture of thousands of different
hydrocarbons and other chemical compounds. In addition, the composition of each is unique and it varies
in different producing regions and even in different unconnected zones. The composition of oil also varies
with the amount of carbon atom present. Significantly, the many compounds in oil differ markedly in
solubility, and susceptibility to biodegradation. Some compounds are readily degraded; others stubbornly
resist degradation; still others are virtually non-biodegradable. The biodegradation of different petroleum
compounds occurs simultaneously but at different rates.
The effect of crude oil and petroleum hydrocarbons on micro algae had been investigated for some decades to address the problem of oil pollution. A major part of the research activities related to oil pollution in recent years has centered on acute and chronic effect of crude oil and specific hydrocarbons on algae. Most of the literature available lay emphasis on the crude oil and hydrocarbons themselves and not on the soluble fractions [1-3]. There is however, scanty literature on the effect of water saturated fractions of crude oil hydrocarbons on the algal community.
The effects of crude oil pollution on growth and metabolic activities of the fresh water algae Chlorella homosphaera and Chlorella vulgaris was investigated by El-Dib et al., (2002) [4]. According to the authors, the presence of crude oil or its refined products in the culture media of algae markedly influenced their growth. Toxicity of the oil was reported to be concentration dependent. Low concentration stimulated growth, protein content and nucleic acid while higher concentrations had inhibitory effect. DNA and RNA responded similarly in crude oil, DNA exhibited more sensitivity than RNA in the two test organisms.
Materials and Methods
Three microalgae were used as experimental microalgae. These were Chlorella vulgaris, Nitzschia palea, and
Anabaena flosaquae [5].
The microalga species was grown in an artificial batch culture prepared according to Chu’s modified No 10
medium [6].
Nine hundred and fifty ml round bottomed transparent glass bottles were used as cultured vessel for both
isolation and in the main experiment.
Unialgal culture of the microalgae used for this investigation was isolated in modified Chu 10 artificial
growth medium from a water sample collected in Benin city. Microscopic examination and identification of microalgae was made using relevant texts [7,8].
The hydrocarbons used (Hexane, Xylene, Benzene and Toluene) were obtained from Thomos Gold venture
in Benin City Edo state.
The WSF was prepared in ratio 1:9 according to the method of Andreson (1974) [9] and Opute (2003) [8].
Various concentrations (0%, 10%, 25%, 50%, 75% and 100%) of water saturated fractions of Hydrocarbons
were prepared using the growth medium and made into replicate. Four hundred and fifty ml each of the
water saturated fractions of the hydrocarbons were measured separately into the experimental vessels. These
were then inoculated separately using 2mls of each of the experimental microalgae with a 5ml syringe. The
experimental bottles were plug with sizeable cotton wool to limit evaporation and prevent contamination
from the environment. The cultures were then place in a southern window for solar illumination.
Absorbance as growth indices was used to evaluate the effect of various water saturated fractions on the
growth of the microalgae. This was read at 745nm using HACH DR 2000 Spectrophotometer using Twenty
five ml aliquot [10a,10b].
Percentage inhibition on the various microalgae was determined using the formular
Where: Theoretical biomass = biomass of control experiment.
Apart from the calculated means, Standard error of mean, Analysis of variance was also calculated to detect
significant difference between the levels of toxic effect of various concentrations of the treatment on the
different microalgae. Where there was significant difference, the Duncan multiple tests was carried out. The
statistical package used was the SPSS version 15.
Results
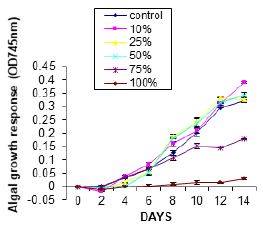
Figure 1 show the growth of Chlorella vulgaris in WSF of Hexane. Growth stimulation was recorded in 0%
to 75% concentrations after an initial lag phase was observed at the early age (day 0 and 4) in the treatment
culture while growth inhibition was recorded in 100% concentration. No significant difference at (p<0.05)
was observed in the growth of the alga in the various concentrations of the treatments.
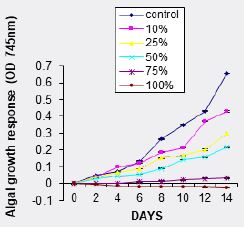
The growth responses of A. flosaquae in different concentrations of WSF of Hexane are shown in (fig 2). The micro alga showed growth stimulation in all investigated concentrations (0% - 100%) after an initial lag phase of growth between day 0 and 2 in higher concentrations (75% and 100%). Total algal growth in the control was higher than that in all investigated concentrations of the treatment. There was no significant difference at (p<0.05) in the growth of the alga in the various concentrations of the treatments.
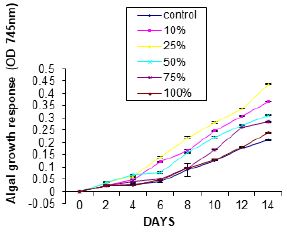
Figures 3 show the effect of WSF of Hexane on growth of Nitzschia palea. An exponential growth of the alga in all concentrations of the WSF investigated was recorded throughout the investigation. There was no visible lag phase in all investigated concentrations rather growth was spontaneous throughout the experiment. Total algal growth in treatment cultures was higher than that in the control experiment.
Discussion
A wide range of hydrocarbons that contaminate the environment has been shown to be biodegraded
in various extreme environments characterized by low or elevated factors such as temperatures, pH and
salinity. Most investigations on phytotoxicity and biodegradation of hydrocarbons have concentrated on the
use of biodegrading microorganisms. Comparatively, little is known about the responses of microalgae to
metabolism of hydrocarbons. Hydrocarbon biodegradation can be significantly stimulated under favorable
nutrient conditions. The increasing number of organism so far reported indicates that there is a growing
interest in the commercial application of hydrocarbon degraders for biological, environmentally friendly
treatment of polluted water. Microorganisms useful for bioremediation must survive and be active under in
situ conditions. In this study, we apply the use of microalgae in biodegradation protocol to determine the
potential of their uses in bioremediation of hydrocarbon pollutants with specific emphasis on Hexane. Our
overall assessment of the responses of the various forms of microalgae showed both growth stimulation and
inhibition at different concentrations. The growth stimulating effect observed in the WSF of hexane at lower
and mid concentration (10% - 50%) could be due to higher mineralization and high rate of evaporation of
the toxic components of the WSF before the termination of the experiment. These findings are in accordance
with the work [11].
The inhibitory effects observed in this study at higher concentrations (75% and 100%) which were pronounce during the exponential growth phase. During this phase, the growth rate of treatment cultures was less than that of the control. However, at the end of the experiment, there was no significant difference (p<0.005) in the growth rate of microalgae in control and low concentration of treatment cultures. This could be attributed to the presence of higher amount of inhibitory substances in this concentration than other concentrations.
Nutrient deficiencies in higher concentrations of treatment cultures could also be responsible. But, without detailed analyses of the nutrient content of the culture media at the end of the experiments, it is impossible to draw firm conclusions. Perhaps the most likely explanation may reside with the presence of more inhibitory substances associated with the WSF at higher concentrations. This finding is further supported by the work of [8,12] who noted that the water soluble fractions from four fuel oil showed considerably different inhibitory effect on growth of six microalgae namely (Agmenellumquadruplicatum, Coccochloriselabens, Dunaliellatertiolecta, Chlorella autotrophica, Cylindrothecasp and Amphorasp). The inhibitory effect in various concentrations could also been occasioned by changes in growth medium due to photooxidation and microalgalphotosynthetic activities. This view is corroborated by the findings of Sargin et al., (2005) [13] who reported that hydrocarbons cause a disruption of the optimal physical state of the cytoplasmic membranes thus compromising the permeability of these membranes to hydrocarbons which in turn facilitate the entry and accumulation of hydrocarbons into the cells.
Conclusion
C. vulgaris showed growth stimulation at 10% - 50% concentrations of WSFs of hydrocarbon while 75%
and 100% concentrations were inhibitory. A. flosaquaes showed growth stimulation n in 10% - 75% of WSF
of Hexane. N. palea showed growth stimulation at all concentrations (10% - 100%) of the WSF of the
hydrocarbon. The inhibitory effects observed in this study at higher concentrations were more pronounced
during the exponential growth phase.
Recommendation
The present study investigated the phytotoxicity and effect of water saturated fractions of Hexane on
growth of microalgae. This work however did not address the effect of nutrient on the process. We therefore
recommend that further work should be carried out with emphasis on the role of nutrient.
Acknowledgements
We are grateful to Prof. Mrs. M.O Kadiri of the University of Benin Nigeria for assisting with the
methodology and some of the facilities used for the research.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!