Biography
Interests
Khalid S. Aljabri, MD, FRCPC, FACP1*, Samia A. Bokhari, MD, SBEM1, Muneera A. Alshareef, MD, SBIM1, Patan M. Khan, MD, MRCP1, Hesham M. AbuElsaoud, MD1, Mohammad M. Jalal, MD1, Rania F. Safwat, MD1, Rehab El Boraie, MD1, Nawaf K. Aljabri, MLT2, Bandari K. Aljabri, MS3 & Arwa Y. Alsuraihi, MS3
1Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia
22Department of Laboratory, Northern Armed Forces Hospital, Hafr Al-Batin, Kingdom of Saudi Arabia
3College of Medicine, Um Al Qura University, Makkah, Kingdom of Saudi Arabia
*Correspondence to: Dr. Khalid S. Aljabri, MD, FRCPC, FACP, Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia.
Copyright © 2018 Dr. Khalid S. Aljabri, MD, FRCPC, FACP, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Albuminuria is a risk factor for cardiovascular disease, and excess urinary albumin is related to
increased all-cause mortality. Patients with type 2 diabetes (T2DM) are almost twice as likely to
have hypertension (HTN) as the nondiabetic population. There have been few reported studies in
national, regional and Asian populations on the prevalence of microalbuminuria in this high risk
population. Therefore, we retrospectively evaluated the prevalence of albuminuria in Saudi patients
with T2DM and HTN.
For the present retrospective study, we analyzed 2315 participants whom are between the age 20 to
98 years. All patients were from the population of the diabetic centre and Primary health centre at
King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia. All data were collected on the basis of
a review of electronic medical data.
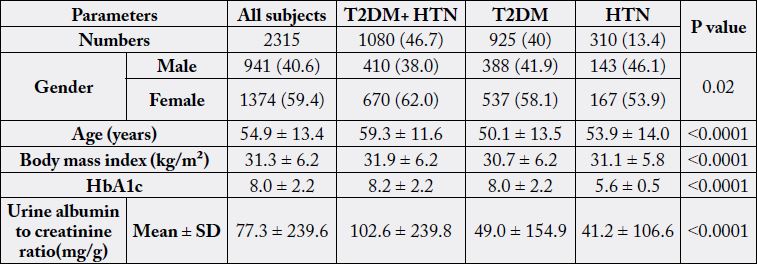
A total of 2315 subjects (the mean age was 54.9 ± 13.4 years, minimum 20 years and maximum
98 years) were included in the analysis. The frequency of female subjects with T2DM and HTN,
T2DM or HTN were significantly higher than male (62% vs. 38% , 58% vs. 42% and 54% vs.
46% respectively, p=0.02). Subjects with T2DM and HTN were significantly older than subjects
with T2DM or HTN Subjects with T2DM and HTN were significantly older than subjects with
T2DM or HTN (59.3 ± 11.6, 50.1 ± 13.5 and 53.9 ± 14.0 years respectively, p<0.0001). Body
mass index in subjects with T2DM and HTN were significantly higher than subjects with T2DM
or HTN Subjects (31.9 ± 6.2, 30.7 ± 6.2 and 31.1 ± 5.8 kg/m2 respectively, p<0.0001). The urine
albumin to creatinine ratio (ACR) in subjects with T2DM and HTN were significantly higher
than subjects with T2DM or HTN Subjects (102.6 ± 239.8, 49.0 ± 154.9 and 41.2 ± 106.6mg/g
respectively, p<0.0001). The frequency of subjects with albuminuria (30-299mg/g) in T2DM and
HTN were significantly higher than subjects with T2DM or HTN Subjects (33%, 23% and 19%
respectively). Moreover, The frequency of subjects with albuminuria (≥ 300mg/g) in T2DM and
HTN were significantly higher than subjects with T2DM or HTN Subjects (7%, 3% and 2% respectively).
There were no significant differences in ACR within each group of T2DM and HTN
or T2DM or HTN in correlation to age groups.
From the present study it is evident that T2DM and HTN is more prevalent than T2DM or
HTN alone in patients with albuminuria. The principal strategy is to slow the progression of renal
disease with aggressive glycaemic control and antihypertensive treatment.
Introduction
Reduction cardiovascular disease (CVD) risk factors such as type 2 diabetes mellitus (T2DM) and
hypertension (HTN) were attempted by many countries [1]. Albuminuria is a risk factor for CVD, and
excess urinary albumin is related to increased all-cause mortality [2].
Patients with T2DM are almost twice as likely to have HTN as the nondiabetic population [3]. T2DM patients may be hypertensive for years prior to the onset of overt diabetes. The prevalence of HTN is further increased in patients with T2DM and renal disease as manifested by elevated urinary albumin excretion rates compared with patients with T2DM and no evidence of renal involvement. The development of diabetic nephropathy is characterized by a progressive increase in the excretion of protein, particularly albumin, an early and continuing rise in systolic blood pressure, and a late decline in glomerular filtration rate, leading eventually to end stage renal failure [4]. Screening for albuminuria and intervention programmes should be implemented early because of the adverse impact of albuminuria on survival in patients with diabetes [5- 9]. Annual screening for microalbuminuria is recommended by the American Diabetes Association [10]. Screening using a semi quantitative dipstick test is simple, and provides immediate and accurate results [11]. There have been few reported studies in national, regional and Asian populations on the prevalence of microalbuminuria in this high risk population [12-19]. These studies have only explored the percentage of microalbuminuria in either patients with diabetes or patients with HTN. Therefore, we retrospectively evaluated the prevalence of albuminuria in Saudi patients with T2DM and HTN.
Methods
For the present retrospective study, we analyzed 2315 participants whom are between the age 20 to 98 years.
All patients were from the population of the diabetic centre and Primary health centre at King Fahad Armed
Forces Hospital, Jeddah, Saudi Arabia. All data were collected on the basis of a review of electronic medical
data. Weight (kg) and height (cm) were measured were recorded. Body mass index (BMI) was expressed
as kg/m2 [20]. Participants were defined as having T2DM according to self-report, clinical reports, use
of antidiabetic agents and HbA1c (≥6.5) [10]. HTN was defined when the systolic blood pressure was
≥130mm Hg and/or diastolic blood pressure was ≥85 mm Hg in addition to receiving any medication for
hypertension [21]. All patients in the present study fulfilled the revised National Kidney Foundation criteria
for the diagnosis of albuminuria [22]. The urine albumin to creatinine ratio (ACR) was used as the index of
urinary albumin excretion. A urine sample was collected during the first morning voiding. Conventionally,
subjects with ACR<30mg/g were defined as having normalalbuminuria (NA). Microalbuminuria (MI) was
defined as 30 ≤ ACR < 300mg/g and macroalbuminuria (MA) as ACR ≥ 300mg/g [22-24]. A polyclonal
radioimmunoassay was used for albumin measurement.
Unpaired t-test analysis and Chi square (X2) test (categorical data comparison ) were used between variables
to estimate the significance of different between groups for demographic and clinical laboratory. All statistical
analyses were performed using SPSS Version 23.0. The difference between groups was considered significant
when P<0.05.
Results
A total of 2315 subjects (the mean age was 54.9 ± 13.4 years, minimum 20 years and maximum 98 years)
were included in the analysis, table 1. The frequency of female subjects with T2DM and HTN, T2DM or
HTN were significantly higher than male (62% vs. 38% , 58% vs. 42% and 54% vs. 46% respectively, p=0.02).
Subjects with T2DM and HTN were significantly older than subjects with T2DM or HTN Subjects with
T2DM and HTN were significantly older than subjects with T2DM or HTN (59.3 ± 11.6, 50.1 ± 13.5
and 53.9 ± 14.0 years respectively, p<0.0001). BMI in subjects with T2DM and HTN were significantly
higher than subjects with T2DM or HTN Subjects (31.9 ± 6.2, 30.7 ± 6.2 and 31.1 ± 5.8 kg/m2 respectively,
p<0.0001). ACR in subjects with T2DM and HTN were significantly higher than subjects with T2DM or
HTN Subjects (102.6 ± 239.8, 49.0 ± 154.9 and 41.2 ± 106.6mg/g respectively, p<0.0001). The frequency
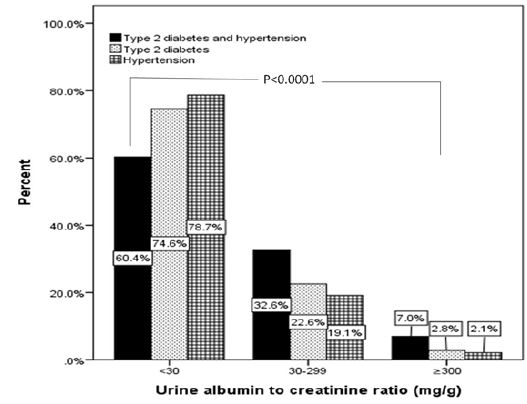
of subjects with MI (30-299mg/g ) in T2DM and HTN were significantly higher than subjects with T2DM or HTN Subjects (33%, 23% and 19% respectively), figure 1. Moreover, The frequency of subjects
with MA (≥ 300mg/g ) in T2DM and HTN were significantly higher than subjects with T2DM or HTN
Subjects (7%, 3% and 2% respectively), figure 1. There were no significant differences in ACR within each
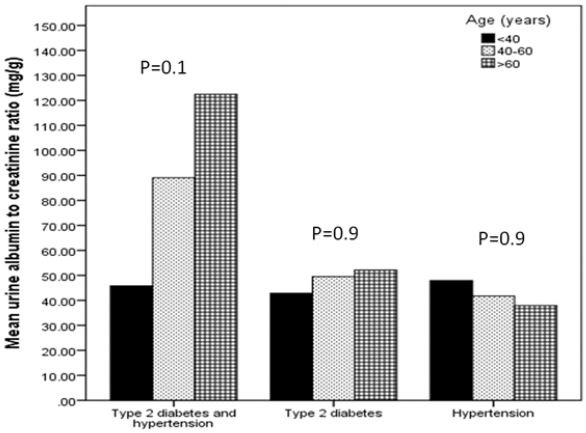
group of T2DM and HTN or T2DM or HTN in correlation to age groups, Figure 2.
Discussion
The major finding of the current study was that albuminuria was frequently associated with T2DM and
HTN subjects compared to T2DM or HTN subjects alone. Moreover, Saudi patients with T2DM and HTN
were more likely to present with MA and MI than patients with T2DM or HTN. MI is the first clinical
sign of diabetic vascular damage and is a predictor for progressive kidney damage, myocardial infarction and
CVD death [25]. Once present, MI progresses over 5-10 years to MA in 22-50% of patients [26,27]. The
development of MA is usually followed by a further decline in glomerular filtration rates [26,27].
The mean difference of HbA1c between T2DM and HTN patients and patients with T2DM was non significantly different (0.18, p=0.07). Hyperglycaemia is an important determinant for the development of proteinuria in patients with T2DM. If instituted early and maintained, effective glycaemic control has been shown to prevent the development of nephropathy and may even lead to regression of albuminuria [28]. Nevertheless, as evidenced by the mean HbA1C of 8.0 ± 2.2%, the majority of patients in this study did not achieve adequate glycaemic control. The mean HbA1C level was significantly (p<0.0001) higher in the macroalbuminuric group (9.2±2.0) than in the microalbuminuric group (8.6±2.2) or the normoalbuminuric group (8.0±2.2). Nonetheless, the high prevalence rate of T2DM and HTN in patients with MI and MA patients reported by us might be reflected by more patients with HTN compared to T2DM or HTN alone. United Kingdom prospective diabetes study (UKPDS) study on T2DM showed that the presence of hypertension is a risk factor for MI and retinopathy and that reducing the incidence of chronic complications was significantly associated with the amplitude of systolic blood pressure decrease, the lowest risk corresponding to a systolic blood pressure below 120mmHg [29].
T2DM remains a tremendous challenge to public health worldwide. T2DM and HTN are common diseases that coexist at a greater frequency than chance alone would predict. HTN in the diabetic individual markedly increases the risk and accelerates the course of CVD, peripheral vascular disease, stroke, retinopathy, and nephropathy [3]. Patients with T2DM may be hypertensive for years prior to the onset of overt diabetes. At the time of diagnosis of T2DM, HTN is found in approximately 70-80% of patients. Still blood pressure rises further in those patients who subsequently develop diabetic nephropathy [30]. Reported prevalence of albuminuria in HTN have yielded variable results due to the chosen cut-off value, patient selection, and more importantly the duration of HTN and of prior treatment. In a cohort of 787 patients aged 18–72 years, a prevalence of MI of 8% was observed [31]. A prevalence of MI of 6% was found in a cohort of 1,041 younger patients (aged 18-45 years) with untreated mild HTN [32]. Both were lower than our report and this finding was also strongly associated with poor glycemic control. Ahmedani et al. showed MI had a higher systolic and diastolic blood pressure compared to microalbuminuria negative group (p<0.001) [33]. Similarly Arkedani et al. and Varghese et al. reported a good statistical correlation between the prevalence of MI and the diastolic blood pressure [34,35]. Pasko et al found that microalbuminuric patients had higher systolic and diastolic blood pressure, suggesting that systolic blood pressure is a significant risk factor for diabetic nephropathy [36]. Svenson et al. showed that high blood pressure increased the risk of developing signs of nephropathy [37].
Despite its complications, T2DM is largely a preventable and treatable disease. Annual screening for MI in all patients with T2DM is recommended, as early treatment that includes CVD risk reduction strategies is critical [10]. The recent availability of MI dipstick test strips provides a Simple method for primary screening and continued monitoring in patients without overt proteinuria. In those who test positive for MI by the dipstick test, quantitative analysis should be carried out to confirm the diagnosis and assist in planning the treatment regimen. The principal strategy is to slow the progression of renal disease with aggressive antihypertensive treatment which should include agents that block the renin-angiotensin system.
Strengths and Limitations
This study was a retrospective and not longitudinal, preventing determination of whether any risk factors
were the cause or result of albuminuria. Moreover, this study was based on hospital based population; thus,
the correct sampling weights were not used for insufficient data, thus limiting the generalization of our
results to the general population of Saudi Arabia.
Conclusion
From the present study it is evident that T2DM and HTN is more prevalent than T2DM or HTN alone in
patients with albuminuria. The principal strategy is to slow the progression of renal disease with aggressive
glycemic control and antihypertensive treatment.
Acknowledgements
We are grateful to the staffs from the diabetic centre and Primary care department at King Fahad Armed
Forces Hospital for their valuable contributions in data collection. The authors have no conflict of interest
to disclose.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!