Biography
Interests
Walnei Fernandes Barbosa1*; Fatima Pereira de Souza2, Evelyn Pedroso Toscano Quintino2 & Giovanni Faria Silva1
1Paulista State University, Departments of Clinical Medicine, Gastroenterology, São Paulo, Brazil
2Paulista State University, Departments of Physics and Multiuser Center Innovation Biomolecular, Molecular
Biology, São Paulo, Brazil
*Correspondence to: Dr. Walnei Fernandes Barbosa, Paulista State University, Departments of Clinical Medicine, Gastroenterology, São Paulo, Brazil.
Copyright © 2018 Dr. Walnei Fernandes Barbosa, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Hepatopulmonary syndrome (HPS) is a clinical relationship between liver disease and intrapulmonary vasodilatations that may result in hypoxemia. HPS may be diagnosed using transthoracic echocardiography (TTE), currently considered the gold standard, or transesophageal echocardiography (TEE) having been described to present greater sensitivity for the detection of bubbles (contrast). The aim of this study was to determine the prevalence of HPS and evaluate the role of contrast TEE in defining diagnosis in the service and to evaluate the role of the TEE in the diagnosis definition. The study admitted patients with liver cirrhosis with different ethology and no primary pulmonary or cardiac disease and a control group of 20 individuals without pulmonary or primary cardiac disease, whose indication of TEE was for stroke in young people to evaluate the presence of patent foramen ovale. Patients and controls were submitted to contrast TTE and TEE, spirometry, chest x-ray and arterial blood gas analysis. Of 59 patients included, 47 (79.7%) were male. Mean age was 48 ± 11 years. Fifteen patients (25.4%) were classified as Child-Pugh A, 30 (50.8%) as B and 14 (23.7%) as C. When TTE was used to detect intrapulmonary vasodilatation, 6 patients (10%) received a diagnosis of HPS, whereas when TEE was used, 8 patients (13.5%) were given this diagnosis (p<0.001). The prevalence of HPS was 10% according to TTE and 13.5% according to TEE. Transesophageal echocardiogram should be performed in patients with chronic liver disease and arterial blood gas abnormalities whenever transthoracic echocardiogram is normal or inconclusive.
Abbreviations (if used)
HPS - Hepatopulmonary syndrome
TTE - Transthoracic echocardiography
TEE - Transesophageal echocardiography
Introduction
Hepatopulmonary syndrome (HPS) is a clinical relationship between liver disease and intrapulmonary
vasodilatation that may result in hypoxemia [1-3] or in an increased alveolar- arterial oxygen gradient [4].
This syndrome is found in around 17.5% of liver transplant candidates [5] and in around 10% of patients
with cirrhosis [6]. A large variation in its prevalence is reported in the literature depending on the diagnostic
method used(contrast echocardiography, lung perfusion scintigraphy or pulmonary angiography) and on the
cut-off limit established as a reference for the definition of hypoxemia and/or an increase in the alveolararterial
oxygen gradient, as well as the selection of the study population [7-9].
Various forms of treatment have been used in HPS; however, results have either been unsatisfactory or the conclusions reached were that further clinical trials would be required to clarify the significance of findings [10].
The causes of HPS are unknown; however, it is believed to be the result of an imbalance between vasoconstrictors and vasodilators such as nitric oxide and/or between vascular cell growth inhibitors and vascular cell growth promoters such as hepatocyte growth factor and vascular endothelial growth factor [11,12]. Pulmonary vascular changes may occur both as precapillary and capillary vascular dilatations varying from 15 to 500μ in diameter or as arteriovenous communications located in the gas exchange units [4].
In order to reach a diagnosis of HPS, intrapulmonary vasodilatation must be confirmed. Contrast echocardiography is currently considered the non-invasive procedure of choice for detecting an intrapulmonary shunt [13], and constitutes the most appropriate method for the screening and identification of the vascular changes present in HPS [14,15].
Transthoracic echocardiography (TTE), currently considered the gold standard, or transesophageal echocardiography (TEE) may be used for this purpose. Since the sensitivity of TEE is higher, the simple presence of contrast in the left cavities does not constitute a criterion for the diagnosis of intrapulmonary vasodilatation and must be classified in accordance with the amount of contrast in the left atrium [16]. On the other hand, since sensitivity is greater, it may be possible to diagnose HPS at an earlier stage, resulting in the patient being referred for a liver transplant at a more opportune moment, consequently improving outcome. Mortality rates related to liver transplant have been found to be higher when HPS is more advanced [17]. Rates of up to 30% in 90 days were described when PaO2 was < 50mmHg at pre transplant evaluation or when a response to 100% oxygen was not achieved (< 300mmHg) and when extra pulmonary uptake in the brain was greater than 30% at lung perfusion scintigraphy [18]. Moreover, Vedrinne et al. [16] and Aller et al. [19] reported uncertain results using transthoracic echocardiography that could have been evaluated better using transesophageal echocardiography. The objectives of this study were to define the prevalence of HPS in the population of patients with cirrhosis and to evaluate the role of contrast transesophageal echocardiography in the diagnosis of this condition.
Materials and Methods
The total of 79 individuals were evaluated, with the control group composed of 20 individuals and 59
patients with cirrhosis of the liver of different etiologies, who had no primary pulmonary or cardiac disease
as indicated by their clinical history, physical examination, chest x- ray, spirometry and echocardiogram,
were enrolled to the study. Arterial blood gas analysis was performed by radial artery puncture, with the
patient in the supine and seated positions for 1 minutes prior to the procedure. Hypoxia was defined as
PaO2< 70mmHg and an increased alveolar-arterial oxygen gradient ≥ 20mmHg. Orthodeoxia was defined
as a decrease > 3mmHg in PaO2 when the patient moved from the supine to the seated position. The
calculation was performed using the equation 1:
in which:
A-aDO2 = difference A-a in mmHg;
%O2 air = % of oxygen in inspired air;
Patm = atmospheric pressure in mmHg;
pO2= partial pressure of arterial oxygen in mmHg;
pCO2 = partial pressure of arterial CO2 in mmHg.
The patients and the control group were submitted to contrast transthoracic echocardiography (TTE) followed
by contrast transesophageal echocardiography (TEE). Control group were selected from non-hepatopathy
patients, those with a history of stroke and who were undergoing transesophageal echocardiography to rule
out Patent Foramen Ovale. Therefore, the TEE had already been requested and scheduled independently of
the study.
In view of the higher sensitivity obtained with transesophageal echocardiography, subjective graduation was used, as previously described in the literature [16], modified for the present study as follows: grade 0 (absent): an absence of bubbles in the left cavities; grade 1 (minimal): very few punctiform microbubbles; grade 2 (mild): some bubbles present but the cavity is not filled homogenously; grade 3 (moderate): many bubbles, totally filling the cavity, density being less than that found in the right atrium; grade 4 (significant): many bubbles, totally filling the cavity, with density similar to that of the right atrium. The exams were recorded and later evaluated by a second observer. The observers were blinded with respect to the Child- Pugh classification of the patient and to the results of arterial blood gas analysis. The second observer did not have access to the results of the TTE or TEE exams as reported by the first observer. The result considered to be final was that given by the first observer, who performed the exams.
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was previously approved by the Internal Review Board of the São Paulo Hospital of the Federal University of São Paulo. All patients signed an informed consent form.
Statistical analysis was performed using Pearson’s chi-square test to verify the relationship of dependence
between dichotomized variables and Fisher’s test whenever applicable. The kappa coefficient of reliability
was used to measure agreement between two evaluations grouped into categories. Significance level was
defined at 5% (p<0.05) throughout the statistical analysis.
Results
Fifty-nine patients were included in the study, of which 47 (79.7%) were male. Mean age was 48 ± 11 years.
Fifteen patients (25.4%) were classified as Child-Pugh A, 30 (50.8%) as B and 14 (23.7%) as C. The control
group was composed of 20 individuals, 11 of which (55%) were female. Mean age was 51 ± 19 years. In 21
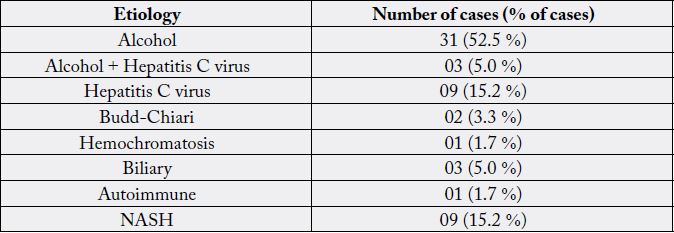
patients (35.5%), cirrhosis of the liver was confirmed by histology. Among the results obtained the most
prevalent etiology showed that 52.5% affected by alcohol, 15.2% by hepatitis C and 15.2% by NASH Table
1.
Echocardiograms were performed by a single observer, recorded, classified and later revaluated by a second observer. The degree of agreement found between the two observers was 62% (kappa = 0.616) (p<0.001). TTE was positive for 18 patients (30.5%). All the individuals in the control group tested negative. The results obtained of TEE, all the individuals in the control group were graded 0 or 1. Therefore, TEE was considered negative when patients were classified as 0 or 1 and positive when classified as 2, 3 or 4. Fortytwo patients (71.1%) tested positive. With TTE, a prevalence of intrapulmonary vasodilatation of 30.5% was found, whereas when TEE was used, this prevalence was 71.1% (p=0.001).
Hypocapnia, defined as PaCO2< 35mmHg [20] was found in 44 patients (74.6%). Hypoxemia, defined as PaO2< 70mmHg, was found in 2 patients (3.3%) and orthodeoxia in 19 patients (32.2%). With respect to the alveolar-arterial oxygen gradient, in 51 patients (86.4%) no alterations were found, whereas in 8 patients (13.5%) there was an increase in the alveolar- arterial oxygen gradient of more than 20mmHg, using the equation (1).When TTE, which is considered the gold standard, was used to evaluate the presence of intrapulmonary vasodilatation for the diagnosis of hepatopulmonary syndrome, 6 patients (10%) were found to fulfil the conditions for a diagnosis of HPS. None of these patients were Child-Pugh A, 4 were classified as B and 2 as C (p=0.319).
The results obtained with TEE was used, 8 patients (13.5%) were diagnosed with HPS. None of these patients was classified as Child-Pugh A, 6 were B and 2 were classified as C (p=0.181). Whereas when the results of TTE and TEE were used to diagnose HPS, a prevalence of 10.1% and 13.5% was found, respectively (p<0.001).
Discussions
All the echocardiograms were performed by the same observer, who was blinded with respect to the Child-
Pugh classification of the patients and to their arterial blood gas findings. The exams were recorded and
later revaluated by a second observer who was also blinded with respect to the aforementioned data and
furthermore had no access to the conclusions of the first observer. The degree of agreement between the
two observers was satisfactory (kappa = 0.616) and, although lower than that found by Aller et al. [21], this
present finding confirms that the method is reproducible.
When TTE was used, 18 patients (30.5%) tested positive, indicating intrapulmonary vasodilatation. This finding is in agreement with results reported in the literature, where prevalence rates range from 13% [22] to 47% [23]. None of the patients in the control group tested positive according to TTE, confirming the high specificity of the method, as previously demonstrated by Aller et al. [21]. TEE provided higher image quality compared to TTE. No patient tested positive on TTE and negative at TEE; however, there were no TTE exams in which diagnosis was considered inconclusive. Vedrinne et al. [16] reported greater difficulty in analyzing TTE exams and reported that with this method 8 of the 37 patients (22%) in their series were classified as undetermined, whereas no cases of inconclusive results occurred with TEE. Likewise, Aller [19] described 6 patients with a positive TTE exam who tested negative at TEE, and were therefore considered false-positives. This fact may be explained by the greater difficulty in finding a “good transthoracic window” and to the artifacts that appear in TTE, particularly in obese patients.
These data suggest the superiority of TEE in comparison with TTE for the diagnosis of intrapulmonary vasodilatation. In the present study, when TTE was used for the diagnosis of intrapulmonary vasodilatation, a prevalence of 30.5% was found, while TEE resulted in a prevalence of 71.1% (p=0.001), suggesting greater sensitivity with the latter method.
In the present study 44 patients (74.6%) were found to have hypocapnia, indicating hyperventilation, which is common in cirrhotic patients and is a determining factor for the preferential use of the alveolar-arterial oxygen gradient rather than PaO2, since this method incorporates the partial pressure of carbon dioxide as well as the inspired oxygen level. The high prevalence of hypocapnia found in this study is in agreement with findings reported in the literature [20,24,25]. As for the presence of hypoxemia only 2 patients (3.3%) were affected. Martínez et al. [5] evaluated HPS in 80 patients who were candidates for liver transplant and found hypoxemia, defined as PaO2< 80mmHg, in only 8 patients (10%). Of those diagnosed as having HPS, only 2 (2.5%) had hypoxemia. The prevalence of HPS in that study was 17.5%. Another study carried out to evaluate hemodynamic, pulmonary and arterial blood gas changes following liver transplant in cirrhotic patients [26] reported that in 43 patients with Child-Pugh C cirrhosis, only 2 (5%) had hypoxemia (PaO2< 70mmHg).
Abnormalities in the alveolar-arterial oxygen gradient were found in only 8 patients (13.5%), the great majority (86.4%) having no alterations. This finding is in agreement with other results published in the literature. However, Battaglia et al. [20] studied arterial blood gas abnormalities in 74 cirrhotic patients prior to and following liver transplant and reported alterations in the alveolar-arterial oxygen gradient (≥ 15 mmHg) in 50% of the patients, while 22% of the patients were hypoxemic (PaO2< 80mmHg). Nevertheless, all these patients were awaiting liver transplant, thereby constituting a population with more severe liver disease; moreover, the cut-off limits determined both to define the increase in the alveolar-arterial oxygen gradient and hypoxemia were different from those used in the majority of other studies.
The results of TTE, showed that the prevalence of HPS in the present study was 10.1%, whereas with TEE this prevalence was 13.5%, a statistically significant difference (p<0.001).
Considering that, of the three criteria required for diagnosis of HPS, chronic liver disease constitutes a condition affecting all HPS patients, the sensitivity (S), specificity (Sp), positive predictive value (PPV) and negative predictive value (NPV) for TTE and TEE were evaluated using the presence or absence of alterations in the alveolar-arterial oxygen gradient. While TTE, sensitivity was 75%, specificity 76.5%, PPV 33.3% and NPV 95.1%, whereas with TEE sensitivity was 100%, specificity 33.3%, PPV 19% and NPV 100%. Assuming that it would be more useful to develop a method capable of diagnosing HPS in the initial phases of the disease, hence a more sensitive method, the role of TEE increases in importance in this respect; however, its low specificity leads to a higher risk of false-positive results.
Cirrhosis is considered to be within the spectrum of liver disease and the Child-Pugh score is used to classify its severity. Therefore, there is a strong possibility that intrapulmonary vasodilatations may also occur in accordance with the progression of liver disease. When Child- Pugh classification was evaluated, a statistically significant association was found between the severity of the liver disease and the presence or absence of intrapulmonary vasodilatation (p=0.013), suggesting that the more severe the liver disease, the more intrapulmonary vasodilatations are found. However, when the presence of HPS was correlated with Child-Pugh classification, no statistically significant association was found (p=0.319). This finding may perhaps be explained by the few patients diagnosed with the disease or by the fact that the majority of patients in this sample were classified as Child-Pugh B.
When TEE was used to diagnose HPS and the results were correlated with Child-Pugh classification, no statistically significant association was found (p=0.181); however, when TEE was dichotomized into negative (grades 0 and 1) or positive (grades 2-4), a statistically significant association with Child-Pugh classification was found (p=0.038), thereby reinforcing the relationship between the severity of liver disease and the presence of intrapulmonary vasodilatation. The importance of this study lies in the fact that it defines the prevalence of HPS in our service and evaluates the role of TEE in this syndrome, raising questions and highlighting opportunities for new studies.
One of the principal limitations of this study may be the small control group; however, since TEE is an invasive procedure, its applicability in normal volunteers is consequently restricted.
The results found in this study suggest that HPS encompasses a wide spectrum of manifestations. In earlier stages of the syndrome, there is some degree of intrapulmonary vasodilatation that is sufficient to permit the passage of bubbles but as yet insufficient to cause alterations in arterial blood gas exchange. Patients at this stage of the disease would be those in whom echocardiographyal ready reveals positive contrast but with a normal gradient. A prospective study of patients at this stage would define whether they would develop alterations in the alveolar-arterial oxygen gradient or even hypoxemia, since in the early phases of the disease patients would be able to maintain satisfactory PaO2 levels by increasing their breathing rate and by the consequent development of hypocapnia. As the liver disease progresses and intrapulmonary vasodilatation becomes more significant, this compensatory mechanism would no longer be sufficient to preserve normal oxygenation. With the data obtained from such a study, it would then be possible to explain the significance of intrapulmonary shunt in this group of patients with intrapulmonary vasodilatation, who do not, however, fulfil all the diagnostic criteria for HPS.
It is important to emphasize that TTE depends on the quality of the image and that more artifacts are present with this exam than with TEE. Siostrzoneck et al. [27] reported inconclusive results at TTE in 14.7% of patients with patent foramen ovale. Vedrinne et al. [16] reported 8 cases of undetermined outcomes and four false-negatives when comparing TTE with TEE in 37 patients. Aller et al. [19] also reported difficulty in interpreting TTE due to the poorer image quality of this exam. TEE is superior to TTE in detecting intrapulmonary vasodilatation as concluded in those two studies [16,19]. These results were also confirmed in the present study, since two patients with altered alveolar-arterial oxygen gradient and negative TTE were found to be positive at TEE.
The false-positive was not considered as a possible reason, since the patients presented alteration of the alveolar-arterial oxygen gradient, without presenting pulmonary or cardiac disease that could explain this alteration.
The HPS diagnostic at an early stage of the disease may improve survival of these patients, and future prospective studies should be carried out to evaluate the optimal time for performing liver transplant and its related efficacy. TEE, classified into its different grades, may improve understanding of the pathogenesis and evolution of HPS, defining its prognosis and more effectively evaluating the treatment and reversibility of intrapulmonary vasodilatation.
In clinical practice, since TEE constitutes an invasive method frequently unavailable at healthcare services, which in addition requires the patient to be sedated, it should be carried out only in patients with chronic liver disease and gas exchange abnormalities (hypoxemia or alterations in the alveolar-arterial oxygen gradient) in whom previous TTE was found to be normal or nonconclusive. Therefore, in order to ensure that no delay is incurred in diagnosing HPS, TTE, being a noninvasive method, should be the method of choice for those patients who have not yet developed significant intrapulmonary vasodilatation or significant alterations in gas exchange, who would be the ideal candidates for a liver transplant.
Conclusions
In the present study, the prevalence of SHP was 10% according to TTE and 13.5% according to TEE,
showing that TEE is superior to TTE in the detection of intrapulmonary vasodilation. The TEE should be
performed in patients with chronic liver disease, arterial blood gas abnormalities and whenever transthoracic
echocardiogram is normal or inconclusive. In view of this, TEE could be performed to offer greater
precision in the definition of such cases and would therefore become the gold standard for the diagnosis of
intrapulmonary vasodilatation and HPS. The improvement in the diagnostic sensitivity of SHP allows its
diagnosis at an earlier stage, resulting in the patient being referred for a liver transplant at a more opportune
moment, consequently improving outcome.
Acknowledgements
We thank the patients who took part in the study.
Conflicts of Interests
We have no conflicts of interest to disclose.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!