Biography
Interests
Amgad ElZahaby1, Samy Zaky1, Nawal Hassanain, A.2, El Sayed Radwan3, Mohey Hassanain, A.2*, Mohammed Hegazy1 & Ahmed Maher, M.2
1Tropical Medicine Department, Faculty of Medicine Al-Azhar University, Cairo, Egypt
2Department of Zoonotic Diseases, National Research Centre, 33 EL Bohouth Street, Dokki, 12622, Giza, Egypt
3Leptospirosis Unit- Animal Reproduction Research Institute (ARRI), 5 Hadaek Al-Ahram Street, Giza, Egypt
*Correspondence to: Dr. Mohey Hassanain, A., Department of Zoonotic Diseases, National, Research Center, 33 EL Bohouth Street, Dokki, 12622, Giza, Egypt.
Copyright © 2018 Dr. Mohey Hassanain, A., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Leptospirosis is considered as the most common zoonosis in the world. This study was designed to be a cross section study conducted at the period extending from June 2016 to May 2017 with the aims of determining leptospiral infection prevalence among people living in Shabramant village at El-Giza governorate and identifying associated environmental and behavioral risk factors for the infection. We operated our study on 400 subjects; a structured questionnaire was carried out to collect information on person’s potential risk factors for leptospiral infection and MAT (Micro Agglutination Test) was done using nine Leptospira serovars to determine the presence of leptospiral antibodies and their titers in the sera of investigated subjects. Our results showed that leptospirosis disease was found to be common among studied cases (44%) and L. Icterohemorrhagiae was the only serovar detected among the studied cases. Age and sex can be regarded as risk factors of leptospirosis as middle age female patients were predominating (52.6%). Co-infection was noted in this study; 22.2% of cases had positivity for HCV Abs which can be regarded as a risk factor for acquiring this disease. Also living close to places where rodents and animals inhabit was considered as important risk factor for exposure to such infection. It can be concluded that high prevalence (44%) of leptospirosis was detected among the studied subjects at Shabramant village. Many of the risk factors and environmental drivers identified in our study provide a significant cause for concern about future risk of leptospirosis.
Introduction
Leptospirosis is one of the world’s most wide-spread zoonoses, caused by pathogenic spirochaetes of the genus
Leptospira [1]. Being spread by the urine of animals, the bacteria enter body via abraded skin, conjunctivae or
mucous membranes, after which they disseminate throughout the body. The infection is common in regions
characterized by high rainfall, close contact with livestock or wild animals. Due to increased rainfall and
global warming, leptospirosis is re-emerging, especially in urban areas, where slums are rapidly growing [2].
Incidence of leptospirosis is variable and marked under-reporting is very common. Generally there is a higher incidence in tropical areas. Leptospira is also encountered in a variety of wild and domestic animals but rodents, cattle, dogs and pigs are the predominant hosts in many countries [3]. Different serogroups predominate in different countries but icterohaemorrhagiae is encountered in the majority of countries [4].
The usual presentations of leptospirosis are highly variable [5]. Leptospirosis has been misdiagnosed including other diseases as dengue fever disease, typhoid fever, influenza, encephalitis, yellow fever disease and malaria. But asymptomatic disease is also common (up 60-70%) in endemic regions. Most patients develop a mild febrile illness with rather non-specific symptoms like fever, myalgia and headache. A proportion of patients, however, develop rapidly progressing and severe complications, with a fatality rate up 70% in patients with severe pulmonary complications [6]. Other presentations include pulmonary hemorrhage, iritis, non calculus cholecystitis and myocarditis [7].
Due to the diverse symptoms similar to various other diseases leptospirosis is difficult to be diagnosed clinically alone. So laboratory investigations are required for confirmation. The reference serological one is micro agglutination test (MAT). The used MAT antigens reflect the most prevalent serotypes in the country. Other tests include ELISA and the dipstick test [8].
The available data on the incidence and prevalence of leptospirosis in the Middle East is few [5]. Therefore the purposes of this study were estimating leptospiral infection prevalence among people living in Shabramant village at El-Giza governorate and identifying associated environmental and behavioral risk factors for the infection.
Subjects and Methods
This cross-sectional survey was carried out in Shabramant village in El-Giza governorate, from June 2016
to May 2017. Shabramant with a population of about 60,000 population, located about 20 km southwest
of the Cairo capital city, this village was selected because it is located near streams of water, many villagers
are engaged in work such as vegetable and fruit gardening, livestock farming, fishing, and weaving. Houses
are usually built high-floored on high wooden or concrete poles, with floor and walls of wood or bamboo.
Roofing is of thatch, leaves, and recently of corrugated tinplate. Cattle and buffalo are reared both in sheds
and free range around the village. Livestock, goats, and chickens are also kept by many households, and they
are usually reared free around the houses.
Statistical formula for sample size calculation was considered. The average seroprevalence of T. gondii was
considered as 50% from literature and sample size was calculated as 384 we increased the number of studied
individuals to 400 so we operated our study on 400 subjects after written consent from them was taken.
For all enrolled subjects, who agreed to participate, one individual face-to-face interview was carried out
with each person included in the sample, using a structured questionnaire to collect information on that
person’s potential risk factors for leptospiral infection, such as occupation, ownership of different kinds of
animals, activities associated with water and livestock, and the environmental conditions of the house and
the village. After the interview a blood sample was drawn after signing a consent form approved by the
Ethics Committees of the National Research Centre. Frozen serum samples were sent to the laboratory of
Animal Reproduction Researches Institute for serologic analysis of leptospiral antibodies.
Complete blood picture, PT, liver function tests (ALT, AST, serum albumin, and serum bilirubin; total and
direct), renal function tests (Urea nitrogen and serum creatinine) and viral markers (HCV Ab and HBs Ag).
Statistical Analysis
The data were processed and analyzed using the statistical package for social sciences (SPSS) program.
Statistical tests used were description of quantitative variables in the form of mean and standard deviation
(mean ± SD) and description of qualitative variables by frequency and percentage. Student’s t-test of two
independent samples was used to compare quantitative variables. One way ANOVA test was used to compare
more than two groups’ quantitative variables was carried out by Chi-square test used to compare qualitative
variables and Correlation co-efficient test (r-test) used to rank different variables against each other either
directly or indirectly. Significance level (P) value was > 0.05.
Results
Table (1) shows the socio-demographic characteristics of the studied subjects; mean age of subjects is in late
of thirties, the majority of participants are women (57%). As regard to occupation of the studied subjects
most of them are farmers and house wives. Table (2) represents ecological characteristics among the studied
subjects; 36% have non-concrete ceiling of their houses, 37% are exposed to rodents, 29% show presence of
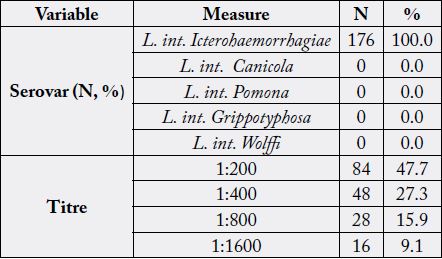
barns & pens in their houses and 37% are in contact with nearby drain/ canal. Table 3 displays prevalence of
leptospirosis among the studied subjects which is found to be 44% and L. int. Icterohemorrhagiae is the only
serovar detected. Analysis of the positive leptospirosis cases regarding socio-demographic and ecological
characteristics of the studied subjects showed the following; Leptospira infection occurred in subjects in
their late thirties (38.0±14.7) and female patients are predominating (52.6%), most of infected persons are
farmers and house wives, positive leptospirosis cases are significantly more frequent among non-concrete
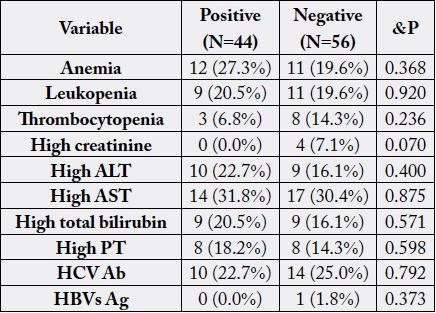
ceiling, exposure to rodents, presence of barns and nearby drain/canal. Table 4 illustrates the impact of
positive leptospirosis on laboratory abnormalities. By studying different risk factors among the studied
samples in regression models, only exposure to rodents was the significant risk factor for leptospiral infection
(Table 5).
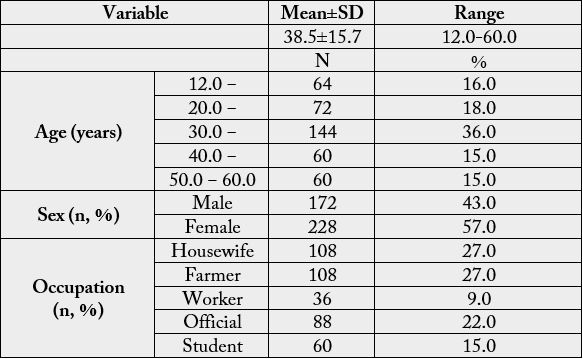
Total=400, Farmer includes all farming activities and farm products sellers, Worker includes all manual workers, Official includes all office-related works, Student include all non-working students
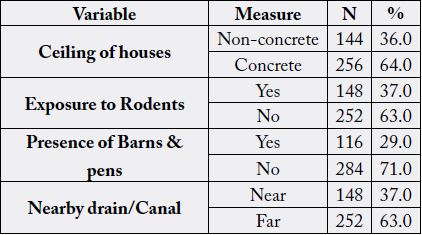
Total=400, Non-concrete includes: floor and walls of wood or bamboo, Roofs are made of thatch and leaves, Rodent exposure is exposure to rodents or its excreta anywhere, Barns & pens include places connected to house to grow up livestock and birds.
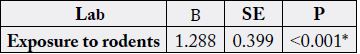
Total=100, β: Regression coefficient, SE: Standard error, *highly Significant
Discussion
Leptospirosis is a worldwide emerging infectious disease of zoonotic importance and large epidemics
and epizootics have been reported all over the globe [12]. In our study, 44% of the studied subjects were
seropositive for Leptospira antibodies. Seroprevalence nearly similar to concurrent work was reported by
many researchers; Laras and Bounlu [13] about 40.9% in Thailand, Al-Robasi et al. [14] 42% in Yemen and
Sohail et al. [15] 40.83% in Pakistan. On the other hand, lower incidence was reported; Kuriakose et al.
[16], 29.6% in a midland rural area of Kerala State, Swai et al. [17], 15% in Tanga City, Tanzania and Reis
et al. [18], 15.4% in an urban slum in Brazil and 37% in Indonesia [13]. However Higher findings were
demonstrated in studies conduct¬ed in Ethiopia about 48% [19], in Tamil Nadu, India about 58% [20], in
Mazandaran Province, Iran was 58% [21] and in Alto Mayo Valley in the Peruvian region of San Martín was 64.6% [22]. Regarding Egypt; lower results were recorded by Maronpot and Barsoum [23] 5.6%, Ismail et al. [24] 24% among patients with hepatitis and Barakat et al. [25] 25.9%. While higher result was reported by Samir et al. [26] 49.7% in Egypt.
In the present study, L. Icterohaemorrhagiae was the only Leptospira serovar observed and 27.3% of the studied leptospirosis cases had a titer of 1:400 and more (1:800 and 1: 1600) reflective of probable recent leptospirosis and further emphasizes the potential public health relevance of serogroup Icterohaemorrhagiae [3]. In Egypt, Ismail et al. [24] reported that L. Icterohemorrhagiae was the major serotype present among the studied patients. Also several investigators recorded that Icterohemorrhagiae was the most prevalent infecting serovar; Juarez et al. [27] in Salvador, Brazil, and Picardeau [28] in mainland, France and French Atlantic and Pacific territories and Alarcón-Villaverde et al. [22] in Alto Mayo Valley in the Peruvian region of San Martín, Peru. On the other hand, the finding of the present study is in contrast with several researches who reported other serovars are most common; Jansen et al. [29] in Germany, serovar Grippotyphosa, Nazrie et al. [30] in Kelantan serovar Betaviae, Khalil et al. [31], in southeast Iran serovar Grippotyphosa and Samsudin et al. [32] in Selangor serovar Sarawak.
In the present study, the demographic factors (Age, Sex and occupation) could be considered as potential risk factors of leptospiral infection; higher prevalence was noted among age group of late of thirties. This finding is similar to results of studies conducted by Yanagihara et al. [33] and Desakorn et al. [34] revealed that most of leptospirosis cases occurred between ages 20-50 years. On the other hand, Anna and Tzimoula [35] recorded that most of leptospirosis cases were among ages of 50-69 years and Kanimozhi et al. [36] suggested that senior age group people (age more than 60 years) are highly proven to this disease probably due to the less immunity factor.
In the concurrent work, our findings showed a significant association between female sex with the seroprevalence of leptospiral antibodies among the studied subjects, as (52.6%) of females were seropositive for leptospiral antibodies while (32.6%) of males were seropositive for leptospiral antibodies (p = 0.045). This is in agreement Padma and Mohammed [37] and Kanimozhi et al. [36]. Our result concerning sex contradicts with other researches who concluded that males are infected with Leptospira than females; Desakorn et al. [34], Al-Robasi et al. [14] and Sakinah et al. [38]. Much more, Vimala et al. [39] found that, the male to female ratio was 1:1, concluded that leptospirosis is prevalent in men and also in women, both are equally affected because of the fact that nowadays both of them are more exposed to the contaminated environment.
In the present work, people in occupations dealing with animals or its products (farmers) are more exposed to Leptospira, and there was statistically significant association among positive cases (p=<0.001). This is in acceptance with findings of Alavi and Khoshkho [40], Lau et al. [41] who concluded that occupational exposure in the agricultural industry is likely to be an important source of leptospiral infection. On the other hand our study is inconsistent with a study conducted by Swapna et al. [42] in Calicut, India, reported that the highest seroprevalence was in hospi¬tal sanitary workers (46%), construction workers (28%), and sewage workers (26%). Much more, Alvarado-Esquivel et al. [43] found that seroprevalence of Leptospira exposure was significantly higher in meat workers (17.7%) than the 4.4% seroprevalence found in waste pickers in Durango City, Mexico.
Concerning the other risk factors among the studied sample in regression models, we found that having nonconcrete ceiling, presence of barns or pens and nearby drain/canal are considered risk factors and exposure to rodents is considered the significant one (p=<0.001).
A systematic review from a selection of 2723 unique publications containing information on leptospirosis, 428 papers dealing with risk factors were identified by Mwanajaa et al. [44], twenty-five studies evaluated exposure to rodents, A majority of the studies were done in South America (10 studies) and on islands (8 studies), concluded that rodents play a major role in transmission of leptospirosis. Lau et al. [41] stated that variables linked with the presence of Leptospira antibodies involved living in villages, working outdoors, living in rural areas, living <100m from a major river and high cattle density in the district.
in our study nonuse of PPE and lack of previous knowledge of leptospirosis collectively were found to be risk factors for acquiring infection. Sakinah et al. [38] reported that the prevalence of leptospiral antibodies was higher (41.2%) among respondents who did not use any protective equipment and they found that respondents in their study who had poor knowledge regarding leptospirosis are higher than respondents who had good knowledge. However, Awosanya et al. [45] found that, wearing of hand gloves, or protective clothing, and boots was not found to be protective against leptospirosis. While De Araújo et al. [46], who revealed that, of the 257 people interviewed, 232 (90.3%) had previously heard about leptospirosis. Much more, Samarakoon and Gunawardena [47], conducted a descriptive study among 460 school students in Sri Lanka, the awareness of leptospirosis was (100%).
In the present study concerning laboratory findings of the studied subjects, our study showed that anemia was present in (27.3%), leukopenia in (20.5%), thrombocytopenia in (6.8%), high ALT in (22.7%), high AST in (31.8%), high total Bilirubin in (20.5%), high P.T in (18.2%) of Leptospira seropositive cases, and no significant differences between leptospirosis positive and negative cases regarding laboratory abnormalities were recorded.
Greene et al. [48] stated that hepatic involvement in leptospirosis can vary from mild liver enzyme elevations with or without hyperbilirubinemia to severe liver failure with signs of hepatic encephalopathy. Das et al. [49] showed that, hemoglobin <10g/dl in 26.3%, leukocytosis>12000/mm3 in 68.4% and 34.2% of the cases had elevated levels of both serum creatinine and ALT/AST. However, Dutta and Christopher [50] stated that, most of the routine laboratory tests showed nonspecific findings.
In the present study, 24% of patients were HCV-Ab positive with insignificant differences between positive or negative Leptospira Ab group. Angnani et al. [51] reported serological evidence of both leptospirosis and hepatitis in 39.4% of their patients. Much more, Das et al. [49] revealed that cases of leptospirosis coinfected with HCV were 5.2% of his patients.
Conclusions
It can be concluded that leptospirosis is an important neglected zoonotic disease despite its high prevalence,
as 44% of the studied subjects at Shabramant village of EL-Giza Governorate were seropositive for leptospiral
antibodies by MAT. L. Icterohemorrhagiae was only detected serovar among the studied subjects.
Symptoms of leptospirosis are not specific, leading to under-reporting of this disease. The main risk factors
of leptospiral infection were exposure to rats, farming, non-concrete ceiling of houses, presence of barns,
pens at houses, presence of nearby drain or canal, lack of PPE, and no prior knowledge of leptospirosis.
Many of the risk factors and environmental drivers identified in our study provide a significant cause for
concern about future risk of leptospirosis. So there is a need to set up reference laboratories for leptospirosis
in specialized hospitals and awareness of the clinicians especially of fever hospital should be increased about
leptospiral infection.
Funding
No funding sources.
Ethical Approval
We operated our study on 400 subjects after written consent form them was taken. Blood samples were
drawn after signing a consent form approved by the Ethics Committees of the National Research Centre.
Conflicts of Interests
The authors declare that they have no conflict of interest.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!