Biography
Interests
Shysil Shafeeq, T. M.1, Feroz Jenner Poolakundan2, Jesha Mohammedali Mundodan3*, Harris, P.4 & Vijay Ashok5
1Department of Nephrology, Malabar Institute of Medical Sciences, Calicut, Kerala, India
2Managing Director & Consultant Physician, Qatar Red Crescent, Doha, Qatar
3Public Health Specialist, MOPH, Qatar
4Associate Professor, Department of Internal Medicine, Hamad Medical Corporation, Doha, Qatar
5Assistant Professor, Department of Internal Medicine, MES Medical College Hospital, Perinthalmanna, Kerala,
India
*Correspondence to: Dr. Jesha Mohammedali Mundodan, Public Health Specialist, MOPH, Qatar.
Copyright © 2021 Dr. Jesha Mohammedali Mundodan, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Thyroid disorders and Diabetes mellitus (DM) are the two most common endocrinopathies encountered in practice. It has long been recognized that thyroid hormones have marked effects on glucose homeostasis but there is a paucity of local studies observing the patterns of thyroid dysfunction in those with type 2 DM. This hospital-based case control study was designed to look for association between type 2 DM and thyroid dysfunction, in a tertiary care teaching hospital in South India, over one year. Data was obtained from 50 individuals above 18 years irrespective of gender, diagnosed to have type 2 DM according to WHO diagnostic criteria and age and sex matched adults without diabetes from bystanders of patients coming to the Hospital.
The mean age of participants was 63.95±10.82 years. There were equal proportion of females and
males in both cases & control groups. TSH values was found to be significantly different (p 0.006)
between cases and controls. But no such statistically significant difference was noted with respect
to F.T3 (p 0.924) or F.T4 (p 0.739). There was significant difference (p<0.001) in proportion of
thyroid dysfunction between cases (54%) and controls (8%). Subclinical Hypothyroidism was
the commonest both cases (32%) and controls (4%). The diabetic subjects had a 13.5 times more
risk for having thyroid dysfunction compared to non-diabetic. There was a statistically significant
difference in the fasting and post prandial blood sugar, HbA1C (glycated haemoglobin) between
the euthyroid subjects and subjects with thyroid dysfunction.
To conclude, there was 13.5 times increased risk of thyroid dysfunction in type 2 diabetic individuals
compared to non-diabetics and most were subclinical hypothyroidism. Hence, it is imperative to
screen diabetic individuals for thyroid dysfunction as early intervention and treatment can help to
achieve good glycemic control and prevent complications.
Introduction
Diabetes mellitus and thyroid diseases are the two common endocrinopathies seen in adult population.
Both conditions tend to occur together in a patient which is attributed to the interaction between the
thyroid hormone and insulin [1]. Thyroid disorder and diabetes mellitus have been shown to influence each
other and an association between them has been reported in literature [2,3]. The first reports showing this
association was published in 1979 [1]. Since then, a number of studies in different countries have been
done to estimate the prevalence of thyroid dysfunction among type 2 diabetic patients. Diabetic patients
have a higher prevalence of thyroid disorders when compared to the non-diabetic population [4,5]. It has
been estimated to be around 10.8% with the commonest manifestation being subclinical hypothyroidism
(50%) followed by hypothyroidism (30%) hyperthyroidism (12%) and postpartum thyroiditis (11%) [6].
There has been a notable increase in the risk of developing thyroid autoimmunity in population with type
2 DM having GAD 65 (Glutamic acid decarboxylase 65) auto antibodies which have also been confirmed
in the pediatric population [7,8]. Prevalence of subclinical hypothyroidism is also noted to be more in
population with metabolic syndrome due to presence of associated obesity, hypertension, insulin resistance
and deranged lipid concentration [9].
In 2007 Shah et al discovered that both thyroid hormone and insulin are involved in cellular metabolism and an excess or deficiency of either one of the two would result in functional derangement of the other i.e. hyperthyroidism can result in hyperglycemia or hypothyroidism results in hypoglycemia [10-12]. Thyroid hormones are known to directly control insulin secretion. The presence of thyroid dysfunction may adversely affect diabetic control. Hyperthyroidism is known to increase the rate of gastrointestinal glucose absorption, increases insulin resistance as well as degradation and in turn worsens the glycemic control of a diabetic subject, while hypothyroidism increases susceptibility to hypoglycemia thus complicating diabetes management [6]. Unidentified thyroid dysfunction could negatively impact diabetes and its complications [3].
On the other hand, DM may also affect the thyroid function to variable extent. DM interferes in thyroid function mainly at two sites; firstly at the level of hypothalamus influencing the TSH release and secondly at the peripheral conversion of T4 to T3. In diabetic individuals in euthyroid state, glycemic status influences serum T3 levels, basal TSH and TSH response to TRH (thyroid releasing hormone) [13]. Poorly controlled diabetes can induce a low serum total and free T3, increases levels of reverse T3(rT3) but may show near normal serum T4 and TSH concentrations by reducing peripheral conversion of T4 to T3. Studies have proven that diabetic control can influence plasma T3 levels; it can also impair the TSH response to TRH or the normal nocturnal TSH peak. The TSH response and ‘low T3 state’ may normalize with good glycemic control but cannot normalize the nocturnal TSH peak in patients with totally absent beta cell function [14].
The diagnosis of thyroid dysfunction in diabetic patients based solely on clinical manifestations can be difficult, as poor glycemic control can produce features similar to hyperthyroidism, such as weight loss despite increased appetite and fatigue. Severe diabetic nephropathy can be mistaken for hypothyroidism as patients with this condition may have oedema, fatigue, pallor and weight gain. Also, symptoms of hyperthyroidism/ hypothyroidism may mimic that of diabetes.
Thus it is important to evaluate diabetic population regarding thyroid diseases clinically or subclinically, as one condition can worsen the other and if left untreated causes diverse complications. Therefore, it is imperative to screen type 2 diabetic population also regarding thyroid status [15]. Screening of thyroid function is mandatory at diagnosis in patients with type1 diabetes due to association of autoimmunity.
Objectives
To study the association between Type2 DM and thyroid dysfunction.
A Hospital based case control study was conducted in the Department of General Medicine of a tertiary
Care Teaching Hospital in North Kerala, South India over a one year period. Adults aged 18 years and
above irrespective of gender with Type 2 DM as per WHO diagnostic criteria attending the outpatient
and in-patient facility of the Hospital were included as cases and age & sex matched adults without
diabetes were taken as controls from among the bystanders of patients coming to the teaching Hospital.
Those not willing to participate, pregnant women, patients with chronic illness other than DM which
can affect thyroid function, patients with known autoimmune disorders which can affect thyroid function,
patients with urine albumin excretion >300mg in 24hr urine sample were excluded [19]. Sample size
was calculated using the formula for testing the significance of difference in mean. Using the formula, n1 = [ z1-α/2 + z1-β ]² [σ1² + σ2²/r] / Δ²; Where r = n2/n1 , r =1, Δ = μ1 - μ2, μ1(mean TSH)19 = 4.58 and
σ1 =2.9; μ2(mean TSH) [16] =3.08 and σ2 =1.56; z1-α/2 = 1.96 and z1-β = 0.80; sample size was calculated
to be 38 cases and 38 controls. It was decided to enroll 50 cases and 50 controls in this study. Consecutive
sampling was used to enroll the cases and controls, starting from the 1st of January 2017. Those age - sex
matched bystanders who consented to participate and reported not having diabetes underwent screening
test (FBS, 2hr PPBS and HbA1c) for diabetes. Those found to be diabetic on screening were excluded. All
the 100 participants were enrolled into the study after taking their informed written consent. All the cases
and controls underwent the following investigations - Thyroid profile (FT3, FT4, TSH), blood sugar levels
(FBS, 2hr PPBS, HbA1C), 24hr Urine albumin. Certain socio-demographic details were also collected
using pre-designed proforma.
Working Definition
Symptoms of hyperglycaemia (polyuria, polydipsia, unexplained weight loss, visual blurring, genital thrush,
lethargy) & raised venous glucose detected once - fasting ≥ 126mg/dl or random ≥ 200mg/dl or Raised
venous glucose on 2 separate occasions fasting ≥ 126mg/dl, random ≥ 200mg/dl or OGTT - 2 h value ≥
11.1mmol/l; HbA1C ≥ 6.5%, but below doesn’t exclude DM.
a) Euthyroid is Normal TSH, Normal F.T3 ,Normal F.T4
b) Hypothyroidism is elevated TSH with Low F.T3 / F.T4
c) Subclinical Hypothyroidism is elevated TSH with Normal F.T3 /F.T4
d) Hyperthyroidism is Low TSH with Elevated F.T3 /F.T4
e) Subclinical Hyperthyroidism is Low TSH with Normal F.T3 /F.T4
f) T3 Toxicosis is Normal TSH with Elevated F.T3, Normal F.T4
g) T4 Toxicosis is Normal TSH with Elevated F.T4 ,Normal F.T3
Data Analysis
Proportion of hypothyroid, hyperthyroid and subclinical conditions were expressed as percentages and
association between thyroid dysfunction and type2 DM was looked for using chi-square test. The significance
in the difference in thyroid hormone levels (TSH, F.T3, F.T4) between type 2 DM and non-diabetic patients
was looked for using ‘t’ test. Correlation between thyroid hormones, blood sugar levels were also looked for.
Source of Funding
The Principal Investigator bore the expenses of the extra investigations done as a part of the study.
Results
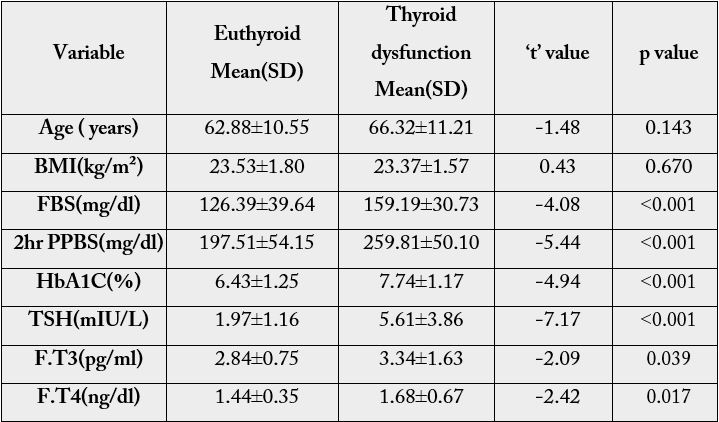
The mean age of 100 participants were 63.95±10.83 years ranging from 45 - 89 years. There was no statistically
significant difference between cases and controls with respect to age and BMI. (Table1) There was equal
proportion of females and males in both cases & control groups. There was statistically significant difference
between cases and controls with respect to FBS (p <0.001), 2 hr PPBS (p <0.001) and HbA1C (p <0.001).
A statistically significant difference (p 0.006) was noted between cases and controls with respect to TSH
values. But no such statistically significant difference was noted with respect to F.T3 (p 0.924) or FT4 (p
0.739) (Table 1).
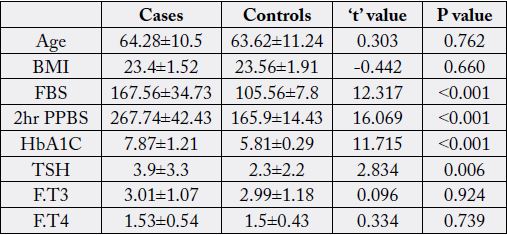
No statistically significant difference was noted between cases and controls with respect to alcohol consumption (p 0.315); smoking (p 0.539); hypertension (p 0.0309); hypercholesterolemia (p 0.444); history of cerebrovascular accident (CVA) (p 0.790) or history of coronary artery disease (CAD) (p 0.220). Hence the cases and controls were comparable in all aspects except diabetic status (Figure 1).
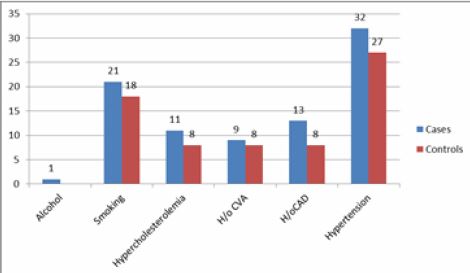
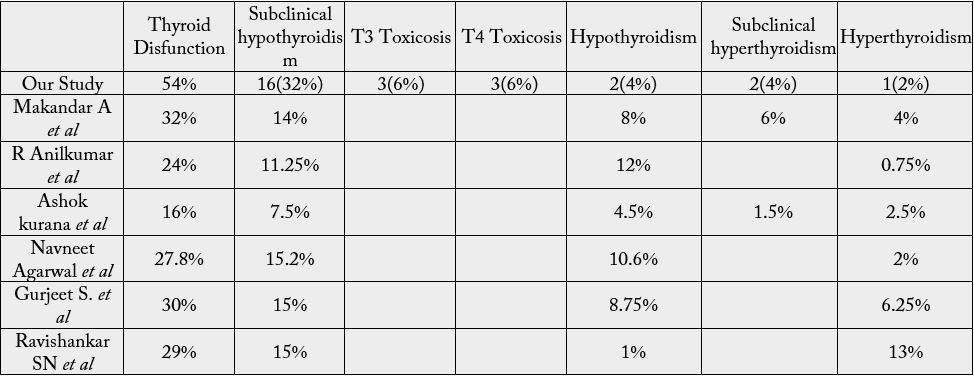
Table 2 shows that of the 100 participants, 69% were Euthyroid and remaining 31% were having thyroid dysfunction, with Subclinical hypothyroidism (18%) being the commonest. T3 Toxicosis and T4 Toxicosis were found in 3% and 4% respectively. Hyperthyroidism, hypothyroidism and subclinical hyperthyroidism were noted in 2% each.
[χ² = 26.556, p <0.001]
More than half the cases (54%) were having thyroid dysfunction while only 8% in the control group were having thyroid dysfunction and this difference in proportion was found to be significant (p <0.001). Among the cases, Subclinical hypothyroidism was the commonest (32%) thyroid dysfunction followed by T3 Toxicosis and T4 Toxicosis in 6% each; Hypothyroidism and subclinical hyperthyroidism constituted 4% each and the least common was hyperthyroidism (2%). Majority of the controls (92%) were euthyroid and only 8% were having thyroid dysfunction. Subclinical hypothyroidism was seen in 4% while hyperthyroidism and T4 Toxicosis 1% each.
Diabetes and thyroid dysfunction was found to be significantly associated
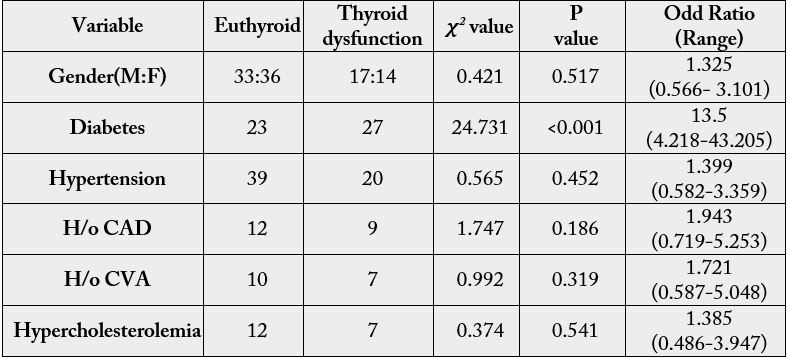
As depicted in table 4, gender (p 0.0517), hypercholesterolemia (p 0.541), hypertension (p 0.452), h/o CAD (p 0.186), h/o CVA (p 0.319) were not found to be significantly associated with thyroid dysfunction. There was no significant difference in age (p 0.143) or BMI (p 0.670) between the euthyroid and thyroid dysfunction subjects (table 4). Smoking was not found to be significantly associated with thyroid dysfunction (p <0.397) though nearly half (14 of 31 with thyroid dysfunction) were smokers while only 25 out of 69 euthyroid were smokers. Thyroid dysfunction and alcohol consumption was not found to be associated (p <0.134).
Discussion
More than half of our cases (54%) had thyroid dysfunction, while thyroid dysfunction was seen only 8%
controls. A similar prevalence of thyroid dysfunction was noted in studies done by Pimenta et al (51.6%),
Bazrafshan HR et al (47.5%), Pasupathi P et al (45%), Swamy RM et al (43%) and Udiong CE et al
(46.5%) in Nigeria [17-21]. In study conducted by Makandar A et al, it was found that, 32% had abnormal
thyroid hormone levels [22]. In our study, subclinical hypothyroidism (32%) was the most common thyroid
dysfunction seen in diabetic individuals. Our results are in accordance with studies conducted by Swamy RM
et al (31.03%), Raghuwanshi PK et al (15%), V. Kayalvizhi et al (21.2%), Vikhe VB et al (14%), Demitrost L
et al (16%) [20,23-26]. A contrast finding was seen in study done by Shaikh AW et al, where hypothyroidism
(35%) was the commonest thyroid abnormality [15].
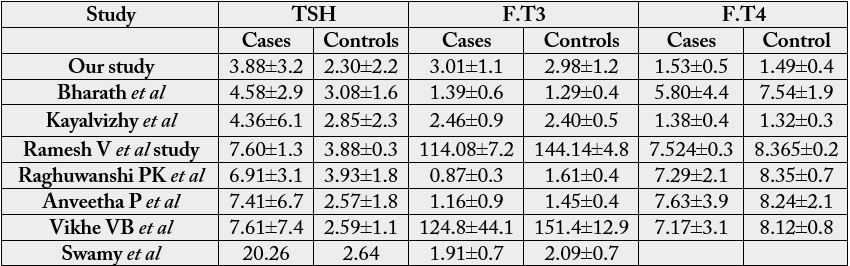
In our study, the mean TSH value of cases was significantly higher compared to controls and the mean F.T3, F.T4 values was slightly higher among the cases compared though not statistically significant. This is in concordance with the study done by V.Kayalvizhi et al [27]. Whereas the studies done by Swamy RM et al, Makandar A et al, Raghuwanshi PK et al, Vikhe VB et al, Anveetha P et al, showed that the level of T3 and T4 were significantly lower while the level of TSH was significantly higher in type2 diabetics as compared to non-diabetics [20,22,23,25,28]. According to Bharat HD et al, the serum TSH level was increased significantly whereas serum T4 level was decreased significantly in diabetic cases when compared with controls [27]. Mean T3 level of diabetic cases was higher than controls but it was insignificant.
In our study a significant association was also found between diabetes and thyroid dysfunction (χ²=24.731; p <0.001). The odds ratio was found to be 13.5 (4.2-43.2), i.e. the diabetic subjects had 13.5 times more risk of having thyroid dysfunction as compared to non-diabetic individuals.
In our study 64% cases had hypertension similar to Sreelatha M et al (50%), Ghazali SM et al in Nigeria (87.5%) and Papazafiropoulou et al in Greece (85%) [29-31]. In our study, no significant association was noted between history of systemic hypertension and thyroid dysfunction similar to studies conducted by Sreelatha M et al and Chubb SA et al [29,32]. There was no statistically significant difference in proportion of hypercholesterolemia between cases (22%) and controls (16%) in our study, similar to the findings in the study by R. Anil Kumar et al [33].
The mean BMI was similar in the euthyroid group and thyroid dysfunction group, similar to findings of R. Anil kumar et al [33]. In our study smoking had no effect on thyroid dysfunction among type2 diabetic patients similar to finding by Al-Geffari M et al [34]. In our study it was found that there was no significant association between thyroid dysfunction and h/o CAD or h/o CVA which is in accordance to the study conducted by Delitala AP et al, Cappola AR et al [35,36].
Conclusion
To conclude, there was increased burden of thyroid dysfunction in patients with type2 diabetes mellitus
compared to non-diabetic individuals. The most common thyroid dysfunction encountered among those
with type2 diabetes mellitus was subclinical hypothyroidism. The odd ratio calculated was 13.5 which
implies 13.5 times more risk in type2 diabetic individuals to have thyroid dysfunction compared to nondiabetic
individuals. So, it is imperative to screen all patients diagnosed to have type2 Diabetes for thyroid
dysfunction and follow up them periodically as early diagnosis and intervention can help to achieve good
glycaemic control and prevent complications.
Limitations
Considering the poor socioeconomic status of the population, lipid profile was not done which would have
helped us to identify other factors which can influence thyroid dysfunction. Due to financial constraints, we
were not able to do thyroid autoantibody assays and Anti GAD 65 antibodies in this population.
Recommendations
In view of current scenario, further researches are mandatory with regards to lipid profile and thyroid antibodies
bearing in mind strong family history and high prevalence of thyroid dysfunction in this population.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!