Biography
Interests
Morshedul Alam
Department of Genetic Engineering & Biotechnology, Bangabandhu Sheikh Mujibur Rahman Maritime University, Dhaka, Bangladesh
*Correspondence to: Dr. Morshedul Alam, Department of Genetic Engineering & Biotechnology, Bangabandhu Sheikh Mujibur Rahman Maritime University, Dhaka, Bangladesh.
Copyright © 2020 Dr. Morshedul Alam. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The novel corona virus SARS-COV-2 outbreak has interrupted the social and global life. During the last century, several human coronaviruses have emerged, causing severe respiratory illness in humans, but the outbreaks were not so devastating. Coronaviruses (CoVs) are a large group of single-stranded RNA viruses belonging to the Coronaviridae family. The world experienced coronavirus for the first time in 2002-2003 by Severe Acute Respiratory Syndrome (SARS) and in 2011 by Middle East Respiratory Syndrome (MERS) and the fatality rate were about 10% and 34% respectively [1], but the biggest concern is that the current SARS-COV-2 outbreak rate already exceeded the previous two terms. The real fact is that though the previous two outbreaks were controlled in a felicitous way, but the present one is still out of control due to lack of appropriate knowledge of its pathogenesis and therapy. Scientists all over the world are trying to get an effective remedy by focusing on vaccine formulation and drug therapy, but a big concern is the efficacy with less side effects and selectivity.
Patients with SARS-COV-2 sense similar symptoms (such as persistent high fever, sore throat, and severe respiratory distress) like the previously emerged outbreaks, like SARS and MERS, the pathogenesis of this disease is more likely akin to that of those coronavirus diseases that involved massive cytokine surge. In addition, the unanimity is that the disease shows severe fatality if the patient’s immune system is already compromised [2].
The host immune system is the tycoon to combat against the invasion of any foreign particles or antigens and in that case, both innate and acquired immunity are involved. In most cases, if the antigen or viral infection is not persistent enough, then the host innate or acquired immunity would able to decline the possibility of pathogenicity. In contrary, in chronic viral infection to lung, patients may experience baneful conformity, including pneumonia that are amalgamated with dysfunctional immune response, i.e, flood of inflammatory cell infiltration and persistent and augmented levels of pro-inflammatory cytokines (IL-1β, IL-6, IL-10, IL-18, MCP-1, TNF-α and so on). These cytokine surges evolve severe immunopathological outcomes that may lead various consequences such as acute respiratory distress syndrome (ARDS), pulmonary edema and multi-organ failure [2].
In general, SARS-COV-2 infects host cells through binding with angiotensin converting enzyme 2 (AEC2) receptor that is predominantly expressed in the pulmonary alveolar epithelial cells in lung [3]. Once get inside into host cells, the virus multiplies by hijacking the host cell machinery as its own and causes damage to the infected cells. This infection and the damaged pulmonary cells persuade local abnormal immune response, which reanimates macrophages and monocytes to respond to the infection. Beside these, to make more vulnerable of the adjacent cells, these inflammatory surge also accumulate oxidants, which insist massive viral infection [4].
Like many other viral infections, SARS-COV-2 also educes their own mechanisms that corroborate their evasion to the host immune system and one such subtlety is the persistent activation of inflammasome, which is encoded by NLRP3 (NACHT, LRR, and PYD domains-containing protein 3). NLRP3 induces caspase 1 activity and pro-inflammatory cytokines such as interleukin- 1β (IL-1β) and IL-18 secretion in macrophages. Inflammasome represents the central regulators of inflammation. Inflammasome activates the inflammation, which is beneficial to viral infection in acute cases but it would be devastative in chronic infection such as influenza A virus infection. SARS-COV-2 also integrates persistent NLRP3 inflammasome activation for its pathogenicity to the host cells [5]. In addition, NLRP3 inflammasome is also activated due to ROS accumulation. So that, at this point, it is plausible that activating anti-oxidant system and suppressing the inflammasome activation would be beneficial to combat SARS-COV-2 infection.
NRF2 is a transcription activator that mediates the inducible expression of antioxidant genes and KEAP1 negatively regulates NRF2. In the living system Keap1-Nrf2 system plays a central role in defense mechanism from oxidative and electrophilic insults. NRF2 is normally ubiquitinated by KEAP1 and subsequently degraded by proteasomes. Inactivation of KEAP1 by oxidative stress or electrophilic chemicals allows NRF2 to translocate and accumulate into nucleus where it binds to AREs to activate transcription of its downstream target genes, which are involved for the synthesis of GSH, NADPH, elimination of ROS, detoxification, inactivation of pro-inflammatory cytokine genes and so on (Fig. 1) [6,7].
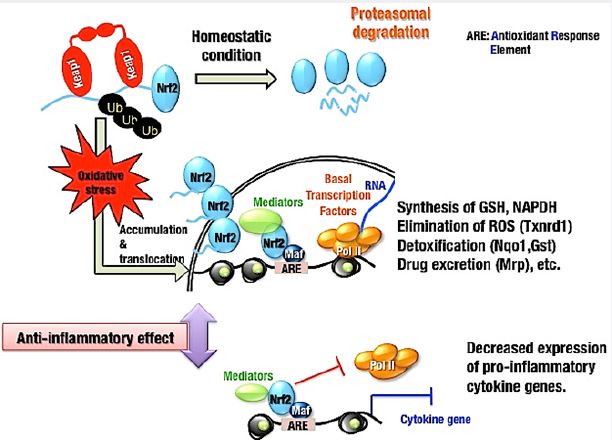
Several studies demonstrated a correlation between NRF2 activation and NLRP3 inflammasome inhibition in many different disease models. Accumulating reports suggested anti-inflammatory effects of NRF2- activating substances with NLRP3 inflammasome inhibition. As the activation of inflammasome is closely related to the interaction with thioredoxin interacting protein (TXNIP), which dissociates from the thioredoxin 1 (TRX1)/TXNIP complex, it was evident that NRF2 acted as a protective regulator against NLRP3 activation by negatively regulating the TRX1/TXNIP complex [8]. NF-κB activation is required for NLRP3 inflammasome priming and specifically, for the induction of IL-1β expression. NRF2 specifically inhibits inflammation-induced transcription mediated by NF-κB. NRF2 directly inhibits Pol II recruitment to the TSSs of the IL-6 and IL-1β genes by binding to the respective gene loci [9]. In addition, it is known that strong Nrf2 activating electrophiles, such as 15d-PGJ2, can also inhibit the NF-κB pathway [10]. There are many substances that activate NRF2 and its downstream target genes, for example, sulforaphane, prostaglandin 15d-PGJ2, curcumin, xanthohumol etc. are the well known NRF2 activators [11]. Sulforaphane also blocks AIM2, NLRP1, and NLRP4 inflammasome activation [12]. Furthermore, sulforaphane also prevents ASC speck formation, which is more likely that inflammasome activiation is inhibited upstream of inflammasome assembly [13]. Thus, depending on available lines of evidence, it can be said that NRF2-dependent NLRP3 inflammasome inhibition should be dependent on NRF2 expression, the induction of NRF2 target gene expression, and a reduction in ROS accumulation.
In summary, based on bunch of evidence, it would be plausible that NRF2 activation that inhibits NLRP3 inflammasome activation and also the suppression of its downstream pro-inflammatory cytokine genes would be considered as a possible therapy for this novel SARS-COV-2 infection and this would also attract scientists to work in depth.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!