Biography
Interests
Igor Ivanov*, Ildar Minulin & Aleksandra Shcheblykina
FSBI “Center for Monitoring and Clinical and Economic Expert Evaluation” of Federal Service for Surveillance in Healthcare - Moscow, Russia
*Correspondence to: Dr. Igor Ivanov, FSBI “Center for Monitoring and Clinical and Economic Expert Evaluation” of Federal Service for Surveillance in Healthcare - Moscow, Russia.
Copyright © 2018 Dr. Igor Ivanov, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
This study is aimed to analyze the results of audits in medical facilities in Russia.
Analysis of the results of audits in medical facilities in Russia.
30 medical facilities implementing Recommendations.
In conditions of the reform of control and supervision in healthcare and realization of the national
strategy “Healthcare development-2020”, the implementation of the effective system of medical
care quality and safety management in medical facilities becomes critically important. In 2015, there were developed Roszdravnadzor’s Practical Guidelines (Recommendations) on management
of the internal system of quality and safety control of medical care in medical facilities. The results
of audits of medical care quality and safety in accordance with the Guidelines identified essential
structural and complex problems in medical care quality and safety management, such as the
absence of unified standards of organizing a system, a low level of competence and knowledge
of healthcare professionals about current approaches to organization, control and management
of medical care.
Introduction
The reform of control and supervision in healthcare has been actively implementing in Russia since 2016.
The reform includes the use of risk-oriented approach when inspectional control is carried out periodically
depending on the category of risk of a medical facility; it also implies the systematization (actualization,
reviewing, removing duplications, and so on) of requirements that come upon medical facilities.
In these conditions, it becomes critically important to implement the effective system of medical care quality and safety management in medical facilities. It is mentioned in the national strategy “Healthcare development-2020” where one of target indicators is the operation of system of medical care quality and safety management in 95% of medical facilities in Russia.
Current challenges have defined a need for developing unified approaches to the management of quality and safety of medical care in medical facilities. Federal State Budgetary Institution “Center for Monitoring and Clinical and Economic Expert Evaluation” of Federal Service for Surveillance in Healthcare developed Roszdravnadzor’s Practical Guidelines (Recommendations) on the internal system of quality and safety control of medical care in medical facilities [1]. The Guidelines were developed with due consideration of the requirements of current world standards for medical practice quality management: Joint Commission International Standards for Hospital (USA), National Safety and Quality Health Service Standards (Australia), Canadian Council on Health Services Accreditation (Canada), and others. The Guidelines became the first document in Russia that combined both most current international requirements and Russian governmental requirements concerning healthcare, taking into account specific character of medical sphere (unlike ISO 9000 standards) [2].
The Guidelines provided the basis for the System of the voluntary certification of medical facilities “Quality and Safety of Medical Care”, which was registered in 2016 (Figure 1). Within the framework of this system, the assessment of conformity of a medical facility is conducted. The assessment of conformity of a medical care is carried out using audits in accordance with the Guidelines. Audit is the most effective tool of assessment and revealing existing problems in a medical facility [3,4].

Methods
The study contains the evaluation of conformity of medical facilities to the requirements of the
Roszdravnadzor’s Practical Guidelines (Recommendations) on management of the internal system of quality and safety control of medical care in medical facilities. We used the results of audits of 30 medical facilities
among which there were district, city and regional facilities from 11 regions of Russia. These facilities are
super specialty inpatient hospitals that deliver both elective and emergency care. The average hospital bed
capacity for district-based facilities is 257 beds, 589 beds for city-based facilities, and 922 beds for regionbased
(republic-based) facilities.
The audits were carried out by multidisciplinary work groups of experts under the supervision of experts from the Federal State Budgetary Institution “Center for Monitoring and Clinical and Economic Expert Evaluation” of Federal Service for Surveillance in Healthcare by the unified procedure based on the Guidelines.
The methodology of the study implies the calculation of the total value of conformity of the medical care in accordance with sections. A checklist containing 10 sections was developed basing on the Guidelines:
The increase of Cronbach’s alpha and combined reliability of the main research structures of 0.7, confirms the reliability of the model’s proper index.
1. Human resources management;
2. Patient Identification;
3. Epidemiologic safety. Preventing and Control of Healthcare Associated Infections;
4. Drug safety. Pharmacovigilance;
5. Control of quality and safety of medical devices circulation;
6. Emergency care in inpatient facilities;
7. Managing clinical responsibility. Patient internal and external transfer;
8. Surgical safety. Prevention of risks associated with surgical intervention;
9. Blood management;
10. Safe environment for the delivery of care. Patient care management. Preventing and managing falls,
pressure injuries.
Each section represented a separate field of quality and safety of medical care assurance and included a list of criteria combined into groups. The assessment system is binary; it determines the conformity or nonconformity to one or another criterion. The non-conformity to any criterion in the group is the reason to consider the whole group of parameters non-conforming.
The sources of information are described in the Figure 2.

Initiators of conducting audits were the authorities of medical facilities. All the members of expert teams adhered to the principles of confidentiality and goodwill. Experts made a point of the fact that the authorities of the medical facilities in question had ensured the personnel that they would not be punished after the audit in any case which made the personnel more open. According to the conditions of the agreement between medical facilities and FSBI CMCEE at Roszdravnadzor the experts had access to all the rooms of the facilities and to all medical and organizational records.
The study was carried out in compliance with ethical standards, taking into account measures for protection of the study subjects’ privacy and the privacy of their personal data. Before interviewing patients, voluntary verbal informed consent statements were received within the framework of the study conducted: the study subjects were informed about purposes, methods, any possible conflicts of interest, expected results, potential risks, and any other important aspects of the study, the subjects also gave their consent to the fact that the received data would be used for research purposes. Before the interview the participants were informed that, the received information would be used without mentioning their names and the names of facilities.
Results
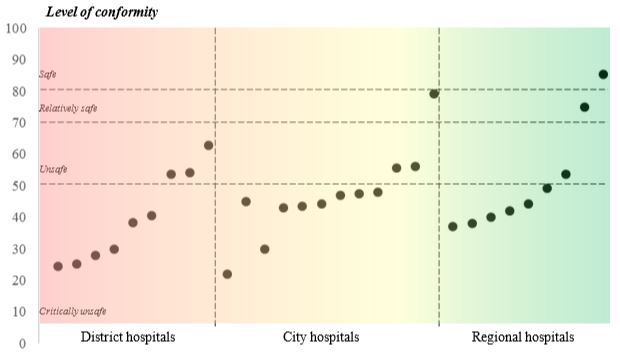
After the audits conducted, the resulting level of conformity of medical facilities to the requirements of the
Recommendations section was 46% (Figure 3). Only in 3 of the 30 medical facilities in question the level
of conformity of the requirements of the Guidelines was over 70%, which highlights the safety or relative
safety of this system (Figure 4). It should be noted that more than a half (21) of the medical facilities in
question shows a critical level of conformity (<50%) to the requirements of the Guidelines.
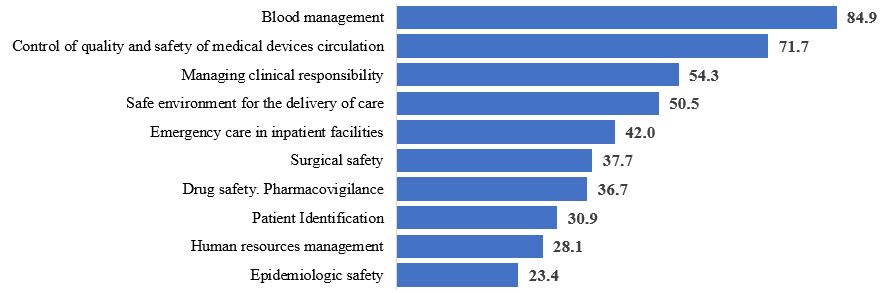
A safe level of conformity (80-100%), which is characteristic of quality and safety of medical care carried out in the given field, was found only for the “Blood management” section (84,9%). Therewith, non-conformities were found in organizational requirements: availability of internal algorithms, personnel’s knowledge and adherence to them, completion of informed consent statements of patients who give their consent to blood transfusion, and non-adherence to algorithms of transfusion of blood and/or its components (more often - fresh-frozen plasma) in terms of clinical justification of indications for transfusion, dosages and adherence to standard operational procedures (carrying out of a biological assay), and completion of documents.
A relatively safe level of conformity (70-80%) was found for the “Control of quality and safety of medical devices circulation” section (average level of conformity is 71,7%). Nevertheless, only 6 of the 30 medical facilities in question had a unified system of control and management of medical devices circulation. For example, one of the investigational medical facilities used a computer program which helped to monitor the condition of the equipment being under repair, functioning equipment, and also terms and results of maintenance, calibration tests, and so on. Medical devices are represented by instruments and equipment with different operational lifetime and maintenance requirements which requires different approaches and skills to work with them. Unfortunately, the personnel of medical facilities do not work properly with instruction manuals of medical devices. The requirements for correct use, maintenance, storage and disposal stated by manufacturers are just partially adhered to in the majority of the assessed medical facilities.
“Unsafe level of conformity” (50-70%) was found for the (7) and (10) sections. An average level of conformity for the “Managing clinical responsibility. Patient internal and external transfer” (7) section was 54,3%. One third of the assessed medical facilities has organizational problems in providing the consistency of healthcare: there are no algorithms for routing patients, for their transfer inside a medical facility (for example, for their transfer to the intensive care unit from clinical units), discharge and transfer to another medical facility (for diagnostic procedures as well). There are also no developed algorithms of organizing external medical consultations, transfer of different categories of patients, transfer of clinical information concerning patients while their transfer, and passing of duty. 26 of the 30 medical facilities have difficulties with communication and transfer of information from nurses to doctors, especially in emergency cases - in case of deterioration of patients’ condition.
The “Safe environment for the delivery of care. Patient care management. Preventing and managing falls, pressure injuries” section has a level of conformity equal to 50,5%. In 22 of the 30 medical facilities in question, there are organizational problems. There is no system of registration of falls and pressure injuries of patients, which means that such cases are not analyzed and the procedure of preventing risks of falls and pressure injuries are not developed, patients and their relatives are not informed about preventing risks of falls and pressure injuries.
“Critically unsafe level of conformity” (less than 50%) was found for (1), (2), (3), (4), (6) and (8) sections.
Within the framework of the “Emergency care in inpatient facilities” section (the level of conformity is 42%), we found out that some medical facilities have no emergency care delivery algorithms that describe the personnel’s actions in certain conditions, and some facilities have no practical system of training the personnel (using simulators) to deliver emergency care, for example, in case of cardio-pulmonary resuscitation and deliver primary care to patients with a shock. The existing clinical algorithms do not conform to current Russian and international clinical guidelines in 26 of the 30 investigational medical facilities. It was found out that one and the same facility might have variable algorithms in different departments. Moreover, there were serious problems with the assessment of practical readiness of the medical personnel to deliver emergency care and give resuscitation: many healthcare professionals rely on the personnel of intensive care unit when such situations occur.
Rather a low level of conformity for the “Surgical safety. Prevention of risks associated with surgical intervention” section is conditioned by very poor results for several requirements: evaluation of risks (of thromboembolism, bleeding, infection and so on) is not agreed upon (by a surgeon and anesthesiologist), informed consent statements are not formally completed (which was confirmed by interviewing patients, during which ½ to ¾ of them could not say what documents they had signed, what intervention they had to undergo, what complications they might have, how long the recovery period would be and what it would be like).
27 medical facilities have not implemented a surgical check-list (e.g., a check-list recommended by the WHO), a “time-out’ rule which gives the opportunity to correct possible mistakes (e.g., misidentification of patients, marking of the surgical site) twice – before administering anesthesia and before making an incision, - or unprepared of the equipment (e.g., a suction unit, pulse oximeter, laryngoscope, and so on). Another problem is an unsafe system of transfer of clinical responsibility for a patient, especially to nurses. In large medical facilities surgeons are engaged in operating rooms during their working days, and at night they admit urgent patients. That is why desk nurses are too busy to care for post-surgery patients, but in the majority of medical facilities such nurses do not have enough knowledge (about alarming or critical values of life factors, such as blood pressure, heart rate, respiration rate, oxygen saturation), do not know algorithms of what to do in case of abnormal values, do not have observation tools (nurse’s observation sheets, check-lists, and so on). Undertriage and ignoring deterioration symptoms that require emergent actions are the reason of returning patients to intensive care units, which were used by experts as triggers to assess the consistency of care.
We could not ignore the issue of customizing approaches to post-operative pain management. The majority of the assessed medical facilities do not use pain assessment scales that is why surgeons’ indications often sound like “…twice a day in case of pain…” while responsibility and operating procedures are not defined. When experts interviewed patients whether they had been satisfied with pain management during a surgical procedure and in a post-operative period using a digital pain assessment scale, about 50% of the respondents were not satisfied with the pain management, and in some cases they estimated the level of pain at 8-9 scores, which cannot be assumed acceptable.
As for the section associated with providing drug safety in medical facilities (the level of conformity - 36,7%), the majority of medical facilities fulfill the requirements concerning control of drugs storage conditions. Though, audits revealed violations of the storage of thermolabile medications, including their storage in resuscitators’ cases (lysthenon, adrenaline). The labeling of vials with infusion solutions did not conform to the established criteria in most medical facilities. There are unsolved problems associated with doctors’ knowledge and taking into account risk factors at drug prescription, including cases of overdiagnosis and polypragmasy. Moreover, it is almost impossible to assess the conformity of the drug selection and dosage to clinical recommendations/treatment protocols as there are no such documents at most workplaces.
As for the “Patient Identification” section (the level of conformity – 30,9%), such a low result may be explained primarily by the fact that healthcare professionals do not accept the possibility of mistakes. Most typically, when experts asked a question: “How do you identify patients when giving them pills, making manipulations and so on?” nurses answered: “I know all of them (patients) by sight. I remember all of them”. Many medical facilities in question had no statutory requirements to use at least two identifiers (full name and birth year) and no developed algorithms of patients’ personalities identification at all stages of healthcare delivery. Most medical facilities had no developed algorithms of identification of patients that are admitted to hospital unconscious with or without documents and attendants. When experts and the personnel discussed the existing practice of patient identification, always some “weak” points were revealed, for example, the absence of voice modules and skills of communication of the healthcare personnel with relatives of patients while informing them about admission a patient in critical condition.
The level of conformity for the “Human resources management” section is 26,5%, and mostly concerns organizational aspects of work of medical facilities in regard to the development of the personnel’s human capacity, use of social-psychological methods of personnel management and a system of effectiveness and performance evaluation. The analysis shows that assessable facilities have some elements of continuous training and development of the personnel, HR records keeping and using administrative methods of personnel management, though the above-mentioned elements do not allow to judge about the availability of a complex approach to personnel management.
According to the WHO’s and other international organizations’ data, the most common cause of death of patients in inpatient facilities is infectious diseases. That is why a low level of epidemiologic safety system management (the level of conformity is 23,4%) is a matter of particular concern, because most healthcare professionals and authorities underestimate risks associated with hospital-acquired infections. The most attention is still paid to adherence to the system of “hospital hygiene and infection control”, including culturing of air, and culturing from surfaces in high security rooms, use of bactericidal lamps, excessive desire to limit visits of relatives in intensive care units, and so on, but not to actual technologies of prevention of healthcare associated infections. All medical facilities had serious violations concerning scrubbing, use of gloves, gowns, and other individual protective devices. There were also registered violations in the process of sterilization of medical devices (excluding medical facilities conforming to current standards), disinfection and disposal of medical wastes. Another common problem is irrational use of antibacterial medications, including the absence or non-adherence to the algorithms of perioperative antibiotic prophylaxis, unreasonable prescription of antibiotics in therapeutic doses (i.e., either prescription of antibiotics 5 days after laparoscopic cholecystectomy, or prescription antibiotics to a child with viral bronchitis).
Conclusion
Thus, if we summarize and analyze the results of external audits of medical care quality and safety in
accordance with the Guidelines, we may highlight that there are essential structural and complex problems
in medical care quality and safety management, which are impossible to solve within one certain medical
facility.
Moreover, the absence of unified standards of organizing a system providing a high level of quality and safety of medical care makes it difficult to manage the system of healthcare at large.
Rather a low level of competence and knowledge of healthcare professionals about current approaches to organization, control and management of medical care is the reason of widely spread archaic approaches to solving problems, searching, defining and punishing a person who is guilty of adverse outcome.
Our practice shows that it is possible to implement in Russian medical facilities a complex system of medical care quality and safety management which includes the requirements of current world standards, is based on conducting audits on a regular basis, and uses comprehensive and process approaches and risk management and patient-oriented principles.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!