Biography
Interests
Demetrio Torres1*, Erick Salcedo2, Laura Díaz3, Miguel Fernández3, Esteban Arellano4 & Mauricio Chandía5
1Medical Doctor, Cancer Center “Nuestra Sra. de la Esperanza”, Pontificia Universidad Católica de Chile, Santiago,
Chile
2Medical Physicist, Cancer Center “Nuestra Sra. de la Esperanza”, Pontificia Universidad Católica de Chile, Chile
3Medical degree, Universidad San Sebastián, Concepción, Chile
4Medical Doctor, Medical School, Universidad Católica del Maule, Talca, Chile. Internal Medicine Service, Regional
Hospital of Talca, Chile
5Medical Doctor, Faculty of Medicine, Universidad de Concepción. Guillermo Grant Benavente Hospital,
Concepción, Chile
*Correspondence to: Dr. Demetrio Torres, Medical Doctor, Cancer Center “Nuestra Sra. de la Esperanza”, Pontificia Universidad Católica de Chile, Santiago, Chile.
Copyright © 2018 Demetrio Torres, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The term autoimmune cytopenias is applied to a heterogeneous group of diseases characterized by a decrease in the peripheral hematological counts of one or more cellular series, product of autoimmunity. Corticosteroids are used in the first-line treatment, while splectonomy and immunosuppresant are used in second-line non-responders. The use of rituximab has been considered in those cases of refractory to first-line treatment or in those who are not candidates for splenectomy.
We present a series of ten patients with refractory autoimmune cytopenias who received rituximab at the regional hospital in Talca, Chile; during the period of 2008-2011. Five patients with immunologic thrombocytopenic purpura were treated; four of them obtained complete response, and one obtained partial response. The median of maximum response time was six weeks, remaining in that category after six months of follow-up care. Four patients were treated with rituximab for autoimmune hemolytic anemia; three of them obtained partial response, while one was lost in follow-up care stage. One patient was diagnosed with Evans syndrome secondary to chronic lymphocytic leukaemia (CLL), achieving considerable increases in the counts of both series, with a sustained response after six months of follow-up care. There were no adverse effects related to the drug.
Introduction
The term “autoimmune cytopenias” (AC) is applied to a heterogeneous group of diseases characterized by
low peripheral blood cell count, product of premature destruction in the reticuloendothelial system, mediated
by autoantibodies. The diseases contemplated in this denomination are: immunological thrombocytopenic
purpura (ITP), autoimmune hemolytic anemia (AHAI) and Evans syndrome [1].
Immune thrombocytopenic purpura is the most common autoimmune cytopenia, with a prevalence of 50 cases per 100000 inhabitants. Its clinical presentation is variable depending on the age and the platelet count, and may be asymptomatic. In children it tends to be acute and with complete spontaneous remission. On the other hand, in adults the course is usually chronic and relapsing [1-4].
In the AHAI the autoantibodies are directed against antigens of the erythrocyte membrane, especially of the Rh system and glycophorin. It can be primary or secondary to neoplasms of the immune system, immunodeficiencies or autoimmune diseases. Its clinical presentation is variable depending on the amount of anemia, and may be manifested as a medical emergency. The prevalence varies between 3-17/100000 inhabitants, according to various reports [1,2].
The association of ITP and AHAI is known as Evans syndrome, being idiopathic in 50% of cases, or secondary to autoimmune diseases or lymphoproliferative syndromes in the remaining cases [5,6]. The clinical presentation is usually manifest and with a greater mortality when it is compared to isolated autoimmune cytopenias.
The first-line treatment in autoimmune cytopenias are usually glucocorticoids; when patients do not respond to steroids or there is corticodependence, second-line treatments such as splenectomy are used, with responses greater than 60%. In case of refractoriness or contraindication of surgery, the use of immunosuppressants or monoclonal antibodies may be considered [1].
Given the frequent presentation of this disease as a clinical emergency, the temporary effectiveness of response to classical treatment and the tendency in many cases to chronicity, several investigations have demonstrated the usefulness of rituximab, as a third-line therapy and even as an alternative to splenectomy in certain patients [6].
Rituximab, a mouse-human chimeric monoclonal antibody with specificity against the B cell CD20 marker, was the first to be approved for therapeutic use. Although its development and primary indication was in B-cell non-Hodgkin lymphomas, its use in multiple autoimmune diseases [7], including AHAI and ITP, is currently known.
Objective
To analyze the effectiveness of rituximab in the treatment of refractory autoimmune cytopenias in adults, at
the Regional Hospital of Talca, during the years 2008 to 2011.
Materials and Methods
A retrospective descriptive study consisting of a series of clinical cases was carried out. The non-random
sample was selected according to the following criteria: over 18 years of age who received rituximab, due to
autoimmune hemolytic anemia, immune thrombocytopenic purpura or Evans syndrome, during the years
2008 to 2011 at the Regional Hospital of Talca, Maule Region, Chile.
Data collection was carried out through the analysis of patients’ clinical records and pharmacy records, considering variables such as: sex, age, comorbidities, previous treatments, clinical evolution, as well as adverse reactions to treatment.
According to criteria established in international publications [8,9], a complete response was considered a platelet count greater than or equal to 100000/ml and absence of bleeding for ITP, as well as an increase to 12g/dL from the baseline hemoglobin concentration and normalization of hemolysis markers, one month after the last dose of treatment, for AHAI (see tables I and II).
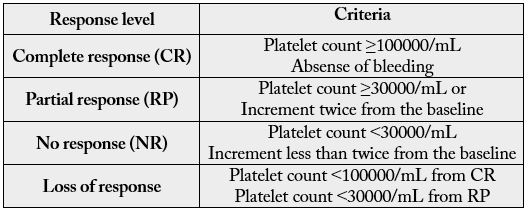
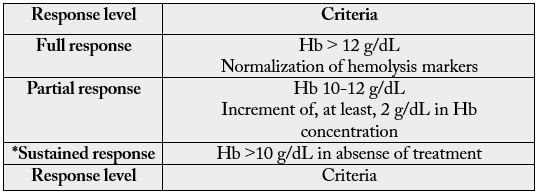
*Duration of response criteria
Results
Five patients diagnosed with ITP received rituximab (Table III). Four cases were idiopathic, while one of them
was considered secondary to generalized lupus erythematosus. The median age of the sample was 48 years.
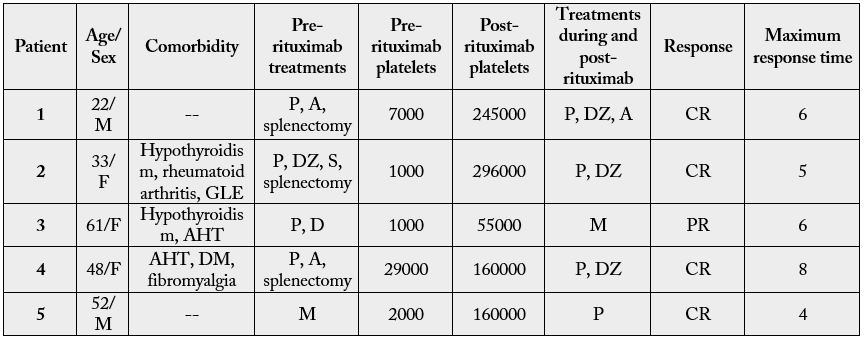
A: Azathioprine; C: Cyclophosphamide; D: Dexamethasone; DZ: Danazol; M: Methylprednisolone; P: Prednisone; AHT: Arterial Hypertension; DM: Diabetes mellitus type 2; GLE: Generalized lupus erythematosus
Prior to use of rituximab, patients received several lines of treatment. All were initially treated with corticosteroids, two of them received immunosuppressants and three were splenectomized. The platelet counts at the start of treatment fluctuated between 1000 and 29000platelets/ml.
Rituximab was administered weekly for four weeks, with a median dose of 600mg per dose. All the patients completed the treatment. In all cases the monoclonal antibody was associated with corticoids.
100% of the treated patients responded to therapy: four of them with a complete response and one with a partial response. The median to reach the maximum response was six weeks.
Of the patients with complete response, 100% presented counts of 150.000platelets/mm3 (between 160.000 and 296.000/ml). 100% continued in full response after six months of follow-up care.
All the patients showed good tolerance to treatment, with no adverse effects being observed.
Four patients were treated for AHAI (Table IV), three of them secondary to lymphoproliferative syndromes
and one to autoimmune disease (generalized lupus erythematosus), all with direct positive Coombs test. The
median age was 55 years. The concentration of hemoglobin at the start of the treatment ranged between 3.4
and 5.9g/dL.
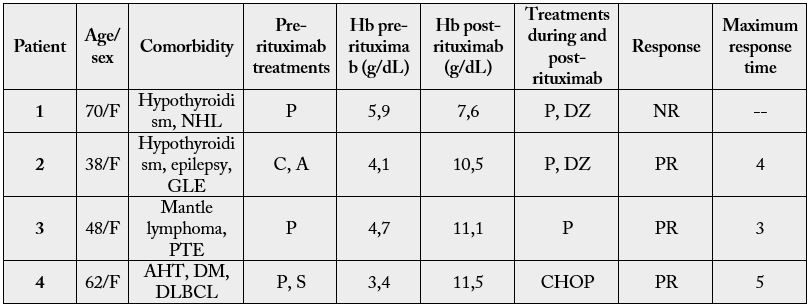
A: Azathioprine; C: Cyclophosphamide; DZ: Danazol; P: Prednisone; S: Solumedrol; AHT: Arterial Hypertension; DLBCL: Diffuse large B-cell lymphoma; DM: Diabetes mellitus type 2; GLE: Generalized lupus erythematosus NHL: No Hogkin lymphoma; PTE: Pulmonary thromboembolism
After the failure of previous treatments, they received a weekly dose of rituximab for four weeks. The median dose administered was 500mg per dose. The 100% completed all four cycles. After one month of therapy ended, three of them (75%) responded partially; while one showed an unsatisfactory response, and abandoned ambulatory control.
One patient studied was diagnosed as Evans syndrome secondary to CLL. Prior to treatment, he presented a platelet count of 4000/ml and hemoglobin concentration of 5.9g/dL. After one month of completing the four cycles of rituximab, the platelet count was 119.000/ml and the hemoglobin concentration was 10.9g/dL. After six months of follow-up care, hemoglobin remained at 13.4g/dL and the platelet count was 279,000/ml.
None of the patients presented adverse reactions during or after the administration of rituximab.
Discussion
The discovery of the usefulness of rituximab in autoimmune cytopenias has meant progress in the treatment
of these diseases after 50 years of minimal advances [2]. Its effectiveness is based on the depletion of B cells
that produce antibodies against the erythrocytes and/or platelets, thanks to their anti-CD20 [10] activity.
International reports show that around 55% of AHAI refractory to conventional therapy present complete remission to treatment with rituximab, maintaining response by varying ranges of time (from 1 year to 22 months) [1,3]. According to this result, 75% of our patients responded partially to treatment, remaining in remission after six months of follow-up care. The time required to get a partial response fluctuated between two and four weeks. The case of AHAI that showed no response to therapy with rituximab was secondary to NHL, so we postulate that this lack of response is due to the large tumor burden that this patient showed.
Studies have demonstrated the efficacy and therapeutic safety of Rituximab in 40-70% of refractory ITPs, with sustained responses for periods greater than one year in 25-30% of them [3]. Consistent with the reports, our series shows a response of 80%, with 60% complete remission and 20% partial response, maintained after six months of follow-up care.
Evans syndrome is also treated first-line with corticosteroids, although exacerbations during treatment are not infrequent, maintaining difficulties in their treatment. There are few reports of cases treated with Rituximab, but remission of both cytopenias has been observed in 50-76% of cases [3,11], encouraging results that are consistent with our observations in the cases described.
Although the dose of rituximab used in this series (see table) is lower than the usually recommended (375m/m2 per dose), there is current evidence that good results occur with the use of a fixed dose of 100mg per week for four weeks, with efficacy comparable to higher doses and a significant reduction in complications and treatment costs [12].
Despite the accumulated experience in relation to the use of rituximab as a second-line therapy in autoimmune cytopenias, there are still no randomized studies comparing it with splenectomy [1,3,13]. Its use as a first treatment in IPT associated with dexamethasone, in a randomized study, showed greater efficacy (although similar rates of relapse) than the use of dexamethasone alone.
We hope to contribute with our report, to the use of this therapy in countries with a similar reality like ours, where access to splenectomy in the public system is limited. On the other hand, we consider it essential to develop randomized controlled studies in this regard, which contribute to understanding the best treatment for this pathological condition.
Acknowledgements
We would like to express our gratitude to Ps. Carla Olguín for her precious support for the development of this article.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!