Biography
Interests
Murillo de Sousa Pinto1,Gabriela Rodrigues de Sousa1, Mônica de Oliveira Santos2, Lilian Carla Carneiro2, Hellen Karine Paes Porto3, Débora de Jesus Pires4 & Aroldo Vieira de Moraes Filho5*
1Academics of the Biomedicine Course of Faculdade Alfredo Nasser, Aparecida de Goiânia, Goiás, Brazil
2Institute of Pathology and Public Health, IPTSP / UFG, Goiânia, Goiás, Brazil
3PhD in progress in Health Sciences by the Federal University of Goiás, Brazil
4State University of Goiás, Brazil
5Institute of Health Sciences / ICS, Faculdade Alfredo Nasser, Aparecida de Goiânia, Goiás, Brazil
*Correspondence to: Dr. Aroldo Vieira de Moraes Filho, Institute of Health Sciences / ICS, Faculdade Alfredo Nasser, Aparecida de Goiânia, Goiás, Brazil.
Copyright © 2018 Aroldo Vieira de Moraes Filho, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
For the maintenance and quality of life of living beings is essential the balance between energy
gain and expenditure. However, obesity is a prevalent health problem worldwide. To combat
obesity many drugs are studied, produced and sold in the worldwide. Sibutramine is a serotoninnoradrenaline
reuptake inhibitor effective in the treatment of obesity. Sibutramine undergoes
metabolism mainly in the liver via CYP3A4, forming two pharmacologically active metabolites
and has several side effects such as irritation, migraine and increased blood pressure. Brazil
is one of the countries that most produces and markets sibutramine and other drugs of this
kind in the world. Our objective was to analyze the mutagenic potential of sibutramine using Allium cepa cells. The selection of the test model was based on criteria such as ease of execution
and rapid reproducibility of tests. The common onion Allium cepa is a very convenient test
system for estimating the harmful effects of chemicals on biological materials. Onion bulbs were
treated with concentrations of 10, 15, 20, 30 and 40mg/L of the drug. Cell samples were
collected and fixed for cytological analysis. The results showed an increasing increase in the
relative abnormality rate (%) of the cells. In addition, we observed aberrations in the divided
and undivided cells, along with cell lysis in samples of concentrations of 30 and 40mg/L of
sibutramine. All experiments were carried out with suitable solutions, new and subjected to the
same conditions of temperature, pH, luminosity and time, so our analyzes of mutation frequencies
and cell division frequencies were expressed on average with results adequate to the standard
deviation. From the data obtained during the development of the study we must insist on the
anticipated need to evaluate the risk / benefit in the prescription of sibutramine, questioning the
well-being and quality of life of the patient, not evaluating this parameter can submit the patient
user to a severe clinical risk making the treatment more extensive and serious complications.
Introduction
When animals receive stimuli, their nerve fibers send signals to the Central Nervous System (CNS), where
the processing, interpretation, elaboration of sensory response or not, memory, associations and response to
the stimulus takes place [1]. Life is essential if the living being obtains sufficient nutrients for the maintenance
of its characteristics and hunger is a stimulus that awakens to this need. Various physiological and / or
psychological stimuli compromise the modulation of hunger leading to the development of disorders or
diseases [2].
The use of drugs that modulate the appetite has been increasing every year. Physiological dysfunctions, anxiety, overwork, agitated life and the need to adapt to a social body pattern stimulate the consumption of these drugs [2,3].
The psychotropic drugs or drugs that act in the control of hunger have as mechanism of action, the alteration of communications between the neurons, leading to the production of diverse effects according to the type of neurotransmitter involved and the way the drug acts. Most are CNS stimulants and inhibit hunger. The use of sibutramine inhibits the reuptake of norepinephrine in a lower proportion of serotonin and dopamine, inducing satiety and increasing thermogenic energy expenditure and consequent weight loss [4].
The metabolism of sibutramine is primarily in the liver and in the presence of CYP3A4 hemoenzyme of the cytochrome P450 enzyme complex which is responsible for terminal oxidases in humans. Sibutramine exerts its pharmacological actions predominantly through its secondary (M1) and primary (M2) amino metabolites, which are inhibitors of the reuptake of noradrenaline, serotonin (5-hydroxytryptamine, 5-HT), and dopamine. Pharmacologically active metabolites M1 and M2 reach maximum concentration in 3 hours, with elimination half-lives of 14 and 16 hours, respectively. The parent compound, Sibutramine, is a potent serotonin reuptake inhibitor. In human brain tissue, M1 and M2 also inhibit dopamine reuptake in vitro, but with a potency three times lower than the inhibition of serotonin or norepinephrine reuptake [5].
Effects on satiety involve central actions on alpha-1 and beta-1 adrenoreceptors, serotonin 5-HT2c receptors, and probably 5-HT2a, which will be excited in the presence of catecholamine class neurotransmitters (Figure 1). Chronic treatment with sibutramine minimizes the adaptive reduction in resting energy expenditure, contributing to the metabolic increase for weight loss [6].
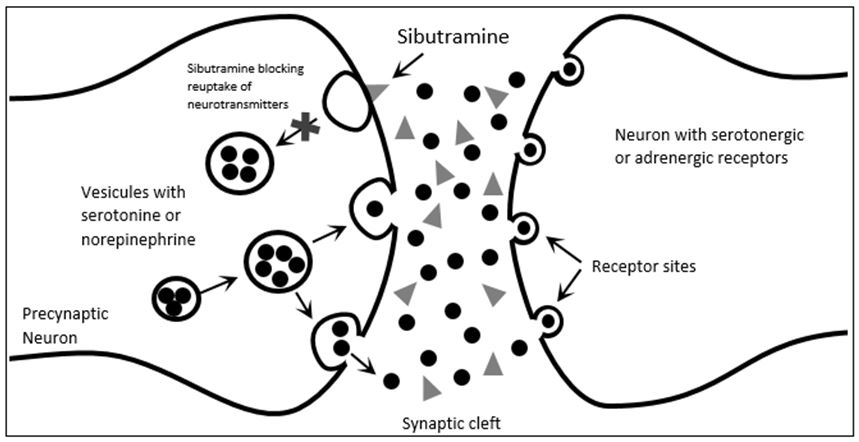
Plasma samples obtained from volunteers treated with sibutramine caused significant inhibition of both noradrenaline reuptake (73%) and serotonin reuptake (54%), but no significant inhibition of dopamine reuptake (16%) [5].
In 2011, the Brazilian National Health Surveillance Agency (Anvisa), through RDC 52, banned the manufacture, importation, marketing and prescription of anorexigenic agents amfepramone, fenproporex and mazindol and established strict criteria for the prescription of sibutramine. According to the Anvisa report the ban was in agreement with the pharmacological and epidemiological arguments about these drugs. By 2011 there were no studies to prove the efficacy and safety of these drugs and their indiscriminate use had grown about 500% from 1998 to 2005 and continued to rise until the effective ban in 2012 [7,8]. Currently, in the interests of the medical profession, RDC 52 was suspended by Law 13,454 of 2017 and even without conclusive studies, anorectics were again produced and marketed in the country [9].
The selection of the test model was based on criteria such as ease of execution and rapid reproducibility of tests. The common onion Allium cepa is a very convenient test system for estimating the harmful effects of chemicals on biological materials [10].
Thus, according to the type of action, the drugs can cause anxiety, drowsiness, excitement, seizures and other adverse effects. Thus, it is difficult to measure the real benefit of a psychotropic in aid of the treatment of obesity. Thus, this work was used the Allium cepa test to evaluate the mutagenic potential, as a possible side effect of sibutramine [11].
Materials and Methods
Organic onion bulbs were purchased locally with reliable source. The dry external scales were removed
without damaging the root area and the central parenchyma of the bud crown was also removed by circular
incision to increase the uptake and uniformity of budding and root growth.
The sibutramine used was commercially purchased with authorization from the Teaching Institution, in the commercial composition of SLENFIG® sibutramine hydrochloride monohydrate, prepared in distilled water, according to the concentration of the samples tested.
For the positive control of the test, Paracetamol/Generico® (C8H9NO2) NeoQuímica was used, commercially purchased with authorization from the Teaching Institution, prepared in distilled water, according to the concentration of the samples tested.
The Allium cepa strain bulbs were washed in running water for about 30 minutes. Carefully, the roots of the
bulbs were placed on exposed glass beakers to prevent light from entering, so that only the central parenchyma
of the bud crown was in contact with the samples. For each sample analyzed, five onion bulbs were used and
placed in contact with the drug concentrations for 24 hours. The negative control was performed in the same
way using distilled water [12,13,14].
The standard sibutramine test concentrations for the experiments were 10mg/L, 15mg/L, 20mg/L, 30mg/L and 40mg/L. These concentrations were selected based on the dosages considered subdose (10mg, where the dose described is lower than the dose at which the drug reaches therapeutic effect), therapeutic dose (15 and 20mg) until overdose (30 and 40mg where the dose described is higher to the dose at which the drug achieves therapeutic effect, being able to reach toxicological effect) [15,7].
The positive control was Paracetamol® at 800mg/L concentration. After growth, the roots immersed in the samples were measured and then fixed in Carnoy’s solution (acetic acid and ethyl alcohol, in the concentration of 3: 1) for 12 hours. After fixation, the roots were washed in distilled water for five minutes and stained on slides. For that, the roots were stained with acetic orcein dye in the dilution of 2% orcein in 45% acetic acid. The root tips were cut and heated for one minute in counting with the dye. Then the roots were placed on slides covered by coverslips and one drop of acetic orcein dye was added between slide and cover slip. Subsequently, the root was crushed by gentle pressure. The observation of the slides was performed under an optical microscope, with a 100x objective, counting 5,000 cells, observing the mitotic indexes and the chromosomal and mitotic changes [12,16,17].
The statistical test used was ANOVA OneWay followed by Bonferroni posttest with variation <0.05 (p <0.05), performed using the statistical program GraphPad Prism v 5.0.
Results
All experiments were carried out with suitable solutions, new and subjected to the same conditions of
temperature, pH, luminosity and time, so our analyzes of mutation frequencies and cell division frequencies
were expressed on average with results adequate to the standard deviation.
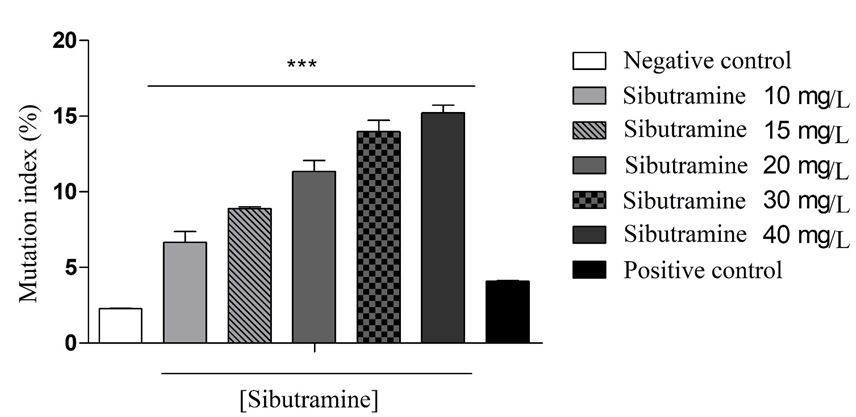
The analysis of the mutation frequencies of the sibutramine treatments expressed a significant difference when compared to the negative control, showing an increase in the mutation index according to the administered dose, which calls into question the warning in the abusive use of sibutramine, since this ascendancy of the quantity of mutant cells at all concentrations, especially at concentrations of 30mg/L and 40mg/L, are remarkably important in inferring an additional concern about the administration of the drug at high doses (p <0.001).
Analysis of the cell division frequencies did not present a statistical difference between the treatments and the controls (Figure 3), contrary to what happened in the study with the drug Orlistat®, which is also a drug used for weight loss, according to [18]: “Regarding the Mutation Index (MI), when comparing the negative control and treatments, at three concentrations the drug significantly reduced MI at the two lowest concentrations of the drug.”
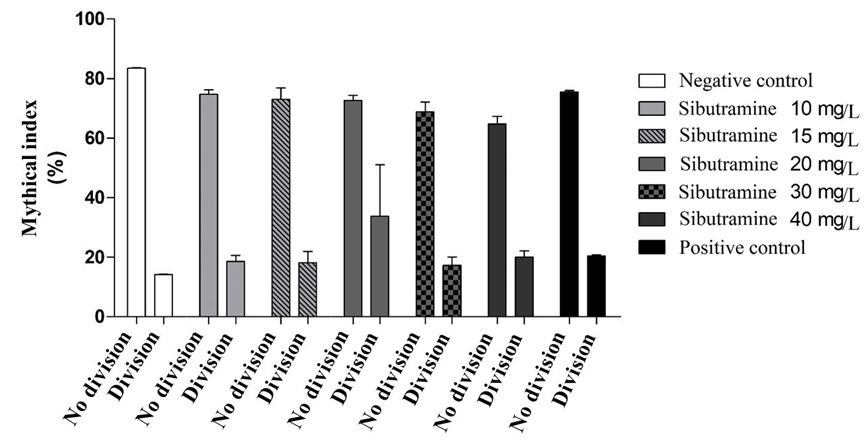
Figure 2 shows a balance in the mitotic index between the cells that were not in division and the cells in division in the treatments and controls whose function is to show and validate the cellular count with minimum standard deviation.
Discussion
The mutation is a consequence of DNA damage and this may be the initial stage in the process by which
most chemicals begin to form a tumor and that mutations can occur in both germ cells and somatic cells and
depending on the genes may or may not have phenotypic effects [19,20].
One of the greatest difficulties encountered in the execution of the study was related to the treatment with 30mg/L and 40mg/L of sibutramine, since there was a higher incidence of cells with chromosomal aberrations, a good part of the cells that would be analyzed presented in a state of lysis, extravasation of the nuclear material, cytoplasmic and nuclear morphological irregularity in addition to the formation of more than one nucleus per cell, as for cytotoxicity it was possible to observe to the naked eye the change in consistency and color of the reticular roots in an apparently healthy and whitish gelatinous color, fragile and slightly hyaline color.
A possible explanation for the genotoxicity and consequent lysis of the A. cepa cells observed may be the indirect action of sibutramine through the induction of oxidative stress, mainly mitochondrial, and the generation of reactive oxygen species (ROS), allowing free radical binding to proteins and DNA in A. cepa. The presence of sibutramine with 30mg/L and 40mg/L probably triggered an imbalance of membrane potential, calcium and sodium flow and consequent lysis of organelles and membrane / cell wall in A. cepa [21].
Some authors tested the genotoxic activity of sibutramine in Swiss (Mus musculus) cells and evaluated their mitotic index and possible genotoxicity through the comet and micronucleus test. The concentrations tested ranged from 5 to 40mg/kg. The results obtained point to a potential mutagenic role of dose-dependent sibutramine and the need for further studies and alternative treatments for obesity control [22,23].
Our experiments showed a balance in mitotic index (Figure 2) between non-dividing cells and dividing cells in treatments and controls as did the test performed in the cytotoxicity and mutagenicity investigation of the hypocelestermant simvastatin “the IM obtained for the Simvastatin concentrations in this test system were not statistically different when compared to the respective cell division indices of their respective controls and recoveries. Also they were not significantly different from the MI of the negative control group, in the respective times of samplings “[24].
Conclusion
From the data obtained during the development of the study we must insist on the anticipated need to
evaluate the risk/benefit in the prescription of sibutramine, questioning the well-being and quality of life of the patient, not evaluating this parameter can submit the patient user to a severe clinical risk making the treatment more extensive and serious complications.
Acknowledgment
The authors thank Alfredo Nasser for their research support by providing all the drugs and materials used
in the research.
Interest conflicts
The authors declare that there are no conflicts of interest in the work performed.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!