Biography
Interests
Dr. Vanessa Novaes Barros
Department of Neuroscience/ Neurology, Estácio de Sá University, Brazil
*Correspondence to: Dr. Vanessa Novaes Barros, Department of Neuroscience/ Neurology, Estácio de Sá University, Brazil.
Copyright © 2018 Dr. Vanessa Novaes Barros. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Research for understands human brain and the differences with the other animals happens since many centuries ago. The publication “Origin of Species” in 1859 of Darwin was an important event in the incessant process of searching for the differences and similarities between humans, their close relatives, monkeys and other animals. However, our particularities have made us so unique that the British paleontologist and anatomist Richard Owen (1804-1892) published, in the year following Darwin’s publication, his book suggesting a new order for humans (which would be Bimana “those with and a new subclass (Archenchefala, “dominant brain”) that separated it from other primates [1].
Even if this new order and subclass were not accepted, the idea of Darwin was very well established in the scientific community, and from the understanding that we have progeny in common with monkeys, it became very important to anatomically and functionally differentiate the brain of monkeys and other species in order to elucidate the differences in the functioning of the nervous system that give us such refined and unique characteristics.
One of the most interesting subjects of neuroscience now is how the human brain has been expanded. Comparative studies between rodents and primates increasingly point to biochemical relevance, complexity, neural connectivity, histology and functional level to the detriment of anatomical differences. According to these studies [2-8] and primates demonstrate distinct cortical developments such as neural pattern, neurogenesis, axonal growth targeting, synaptic formation, neural maturation among other phenomena controlled by transcription factors, secreted molecules or transmembrane proteins [9].
Regulated gene development has been a candidate responsible for the evolution and development of a primate’s brain. Studies have already shown differences in spatial and temporal gene expression between rodents and primates in relation to a gene that regulates DNA demethylation, Gadd45b, which showed high area-specific expression in the association cortices in the brains of adult whales compared to the brain of mice [9].
The understanding of functional cellular differences between rodents and primates could answer some questions such as: why is our performance in cognitive tasks far superior to non-human primates? And why do non-human primates perform so much better than rodents? What, at the cellular level, determines this performance (upper or lower) of the nervous system? Or are fundamental differences restricted to anatomical sphere dimensions (including new brain areas) and cytoarchitecture? A good way to investigate these issues is to study the pattern of gene or protein expression of the rodent and the nonhuman primate against various stimuli, and to assess whether there are differences between the orders.
It is known from studies in our laboratory that the expression patterns of c-Fos protein are different during neuronal activation induced by a convulsive agent between Wistar rats and marmosets (Callithrix jacchus) in the primary motor cortex, cingulate gyrus and piriform cortex, showing a more enduring expression pattern in primates (results already published - Barros et al., 2015) [10] evidenced by a longer expression peak (1 to 2 hours in rats and 1 to 3 hours in monkeys) and a slower return to basal levels (returning to baseline levels in 6 hours in rats and 9-12 hours in monkeys) of c-Fos protein (Figure 1 and 2).
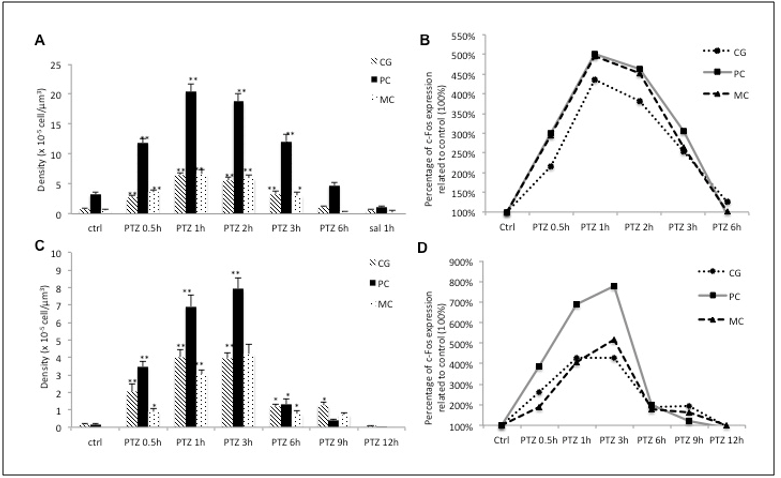
Figure 1: The expression pattern of c-Fos in rats and marmosets. (A) The expression pattern of c-Fos in cingulate gyrus (CG), piriform cortex (CP) and motor cortex (MC) in rats. (B) The relative expression of c-Fos in each of the assessed regions in rats, (C) the expression pattern of c-Fos in CG, piriform cortex (PC) and MC in marmosets. (D) The relative expression of c-Fos in each of the assessed regions in marmoset. *p < 0.05 **p < 0.001 (published in 2015 - doi: 10.3389/fncel.2015.00072)
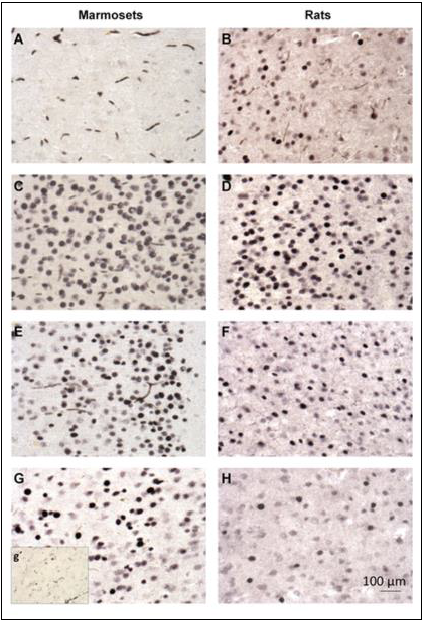
FIGURE 2 | c-Fos immunohistochemistry in the cingulate cortex of marmosets (first column; A, C, E, G) and rats (second column; B, D, F, H). (A, B) ---control group; (C, D)- PTZ 1 h; (E, F)- PTZ 3 h; G-PTZ 9 h; g’- PTZ 12 h; H- PTZ 6 h. (published in 2015 - doi: 10.3389/fncel.2015.00072)
When mapping the c-Jun protein, a distinct expression was again observed in the brains of rats and monkeys, being more durable in rats than in monkeys (results under publication process). Knowing that proteins of the Fos family form dimers with proteins of the JUN family and that this dimer controls the expression of a wide range of genes of late expression, we can consider that the FOS-JUN dimer controls (in the end) the expression of large quantities of proteins that, in turn, define the form, function, and physiology of the cell. With this, this difference becomes extremely relevant for the understanding of brain biochemical processes in rats and monkeys and may be one of the factors for the better brain performance of monkeys. Obviously many other studies are needed to confirm this idea. Concomitant countless other biochemical cascades may be related to this superior primate performance. We are still far from fully understanding of these factors and many other researches that seek to identify these biochemical differences between primates and rodents are necessary and are currently scarce in the literature. But it is certain that the cognitive superiority of a primate must be explained in the future starting from biochemical and not merely anatomical principles.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!