Biography
Interests
Xin Li1, Patrice Berthod1,2* & Estelle Kretz1
1Institut Jean Lamour (UMR 7198), Faculty of Science and Technologies, University of Lorraine, Campus Victor
Grignard, France
2Institut Jean Lamour (UMR 7198), Faculty of Science and Technologies, University of Lorraine, Campus
ARTEM, France
*Correspondence to: Dr. Patrice Berthod, Institut Jean Lamour (UMR 7198), Faculty of Science and Technologies, University of Lorraine, France.
Copyright © 2019 Dr. Patrice Berthod, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Dental cobalt alloys devoted to the constitution of frameworks strengthening fixed partial dentures
may be based on cobalt and chromium. Such alloys generally contain also other elements such as
tungsten, molybdenum and silicon, for example. These minor elements may be added for mechanical
reasons. In contrast with the alloys of the High Noble and Noble categories which are rich in gold or
platinum, these cheaper alloys may be threatened by corrosion. When it happens this phenomenon
may cause health problems, allergic diseases typically. The topic of this work is to characterize the
electrochemical behavior of six alloys based on cobalt and containing 25 wt.%Cr, in a NaCl 9g/L
aqueous solution rated at pH=7.4 and T=37°C. Special focus was done on the effect of the presence of W (2.5, 5 and 7.5 wt.%), Mo (5 wt.%) and/
or Si (1 wt.%). These contents are typical of the ones in commercial dental alloys. The six alloys
were all elaborated by high frequency induction foundry under inert atmosphere. The stationary
electrochemical tests were driven according to the Stern-Geary and Tafel methods, or following
the cyclic polarization technique. Electrochemical Impedance Spectroscopy was also applied. The
results show that the presence of W and its content, on the one hand, and of Si, on the other hand,
have significant effects on the microstructure of the alloys and of their room temperature hardness.
Concerning the electrochemical results the ones issued from the stationary methods are more
interesting that the ones obtained by impedance spectroscopy because of the lack of reproducibility
of these EIS results. The Tafel experiments demonstrated that W did not have any systematic
effect while Mo and Si are beneficial for the behaviour in corrosion. Corrosion resistance is in all
case of high level, anyway, thanks to the sufficiently high Cr content present in all alloys. Thus, no
significant release of cobalt ions is expected in mouth on long time.
Introduction
Metallic alloys are used simultaneously with ceramic materials for constituting many types of dental
prostheses, fixed partial denture for instance. The main reason is a mechanical one: these materials bring the
compulsory strength and toughness (Figure 1). Noble and highly noble metals (palladium, gold, platinum….)
are generally used for the bases of these alloys but these precious metallic materials can be replaced by
much cheaper alloys in some situations. Among these other alloys there are the as-called “Predominantly
Base” ones (“PB”). These PB alloys are the much often nickel & chromium-based ones. However, emerging
alloys based on cobalt are more and more considered to minimize the risks of allergic reactions [1,2]. This
is particularly true if ions are produced in quantity high enough in case of corrosion fast enough affecting
the metallic parts exposed to the buccal milieu. In addition to gold [3] or titanium [4], chromium is often
added to cobalt in significant quantities to obtain Cobalt-Chromium alloys. These ones are thereafter made
more complex with the presence of molybdenum in some cases [5]. Foundry [6] and powder metallurgy
[7] are examples of the elaboration routes allowing obtaining pieces for dental prostheses made of these
Co-Cr-Mo alloys. The use of cobalt-chromium alloys instead nickel-chromium alloys is increasing but their
properties are not so studied as the Ni-Cr based alloys ones even if several published results are actually
available: microstructures [8], mechanical properties [9], corrosion behaviors [10]. However, the interest for
cobalt-based alloys as dental applications (and other medical uses such as surgery) can be illustrated by many
patents pending in the earliest 1970’s (e.g. [11-13]) as well as much more recently (e.g. [14-16]).
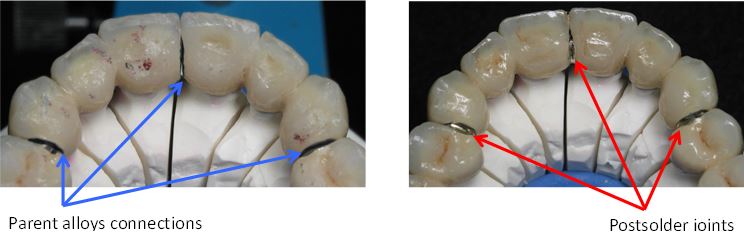
Chromium-cobalt alloys used in dentistry obviously contain elements such as chromium for favouring the resistance against corrosion and other metals as molybdenum to harden the alloys. The first elements may also influence the mechanical properties of the alloys while the later elements may have consequences for the corrosion ones. Furthermore, comparisons to reveal the influences of the elements may be made difficult in case of various elaboration ways for the available alloys. This aim of the present work is, for a given elaboration route (the same used for all the alloys of the study), to simultaneously investigate the influence on microstructures, on mechanical properties and on corrosion behaviour, of the change in content of a present element and of the introduction of additional other elements. The elements of interest which were chosen are tungsten for the influence of its content, and molybdenum and silicon for their additions. The metallurgical state, mechanical properties and corrosion properties which were followed are, respectively, the as-cast microstructures, the hardness specified according to the Vickers technique, and the responses to Tafel experiment and electrochemical impedance spectroscopy.
Materials and Methods
A series of six alloys was chosen for this work. Their targeted chemical compositions are given in Table 1.
These alloys were all elaborated by high frequency induction melting. Cobalt, chromium, molybdenum and
silicon were introduced as small parts of pure element (purity > 99.9%) in the metallic crucible (copper
cooled by internal circulation of water) equipping the furnace (CELES).
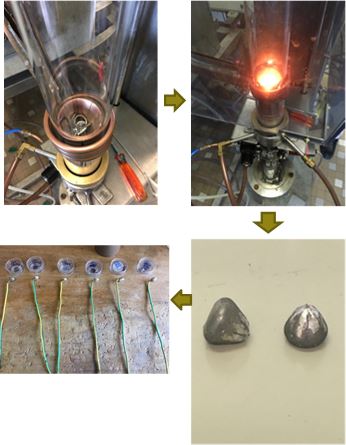
The melting chamber was closed using a silica tube. This one allowed obtaining primary vacuum and filling in pure argon (cycle applied three times), what is compulsory to eliminate oxygen and then to avoid the oxidation of the elements during melting. The silica tube also allowed observing the heating and melting of the liquid alloys. Melting was carried out in a 300 bars Ar atmosphere, by an alternative current circulating in a water-cooled copper coil surrounding the crucible and the tube. The average values of the operating parameters were 110kHz for the frequency and 4kV for the applied voltage. A 5 minutes stage in the liquid state following the heating stage allowed homogenizing the melt. The cooling stage, realized by suppressing progressively the injected power, led to total solidification after about 3 minutes. The total solid state cooling took about 20-25 minutes.
The obtained ingots, all weighing about 40grams and having a compact shape, were cut using a metallographic
saw. By ingot a part was destined to metallographic characterization and indentation (the “metallographic
and hardness sample”, “MH”), and another one to the electrochemical tests (“EC” sample).
Each “MH” sample was embedded in a cold resin system (liquid mixture of resin + hardener). After total stiffening the embedded samples were extracted from their plastic moulds and ground using SiC papers from #240 to #1200 grades). After ultrasonic cleaning and water rinsing, they were polished with textile disk supporting 1μm hard particles. This allowed obtaining mirror-like state for the surface.
The “EC” samples were joined to the denuded part of a plastic-covered electrical wire. For that the extremity of the copper wire was inserted in a slot created using a handsaw and by plastically deforming the wire extremity to secure the electrical contact. The whole (alloy and denuded copper wire) was placed on the bottom of a plastic mould and immersed by the same liquid mixture previously described in the case of the “MH” sample. After stiffening and extraction out of the mould, the obtained electrode was ground and polished until obtaining a mirror-like state, following the same procedure as for the “MH” sample. The “EC” preparation route is summarized and illustrated in Figure 2.
The “MH” samples were observed using a Scanning Electron Microscope (SEM; JEOL; JSM-6010LA) in
Back Scattered Electrons mode (BSE) for examining their as-cast microstructures. The Energy Dispersion
Spectrometry (EDS) device attached to the SEM was used to control the global chemical composition of
these samples.
The same samples were thereafter placed in a Testwell Wolpert indentation machine with which their hardness was measured according to the Vickers technique (Figure 3 left). Per “MH” sample five indentations were performed and the results led to the calculation of the average hardness and of the corresponding standard deviation.

The electrode prepared previously was used as a working electrode associated to a platinum counter electrode
and a Saturated Calomel Electrode (SCE) for the reference in potential. All of them were immersed in a
double-walled three-electrode cell containing a 9g/L NaCl aqueous solution simulating saliva (Figure 3,
right). This one was prepared by dissolving NaCl crystals in distilled water. Its pH was adjusted to 7.4 by
addition of diluted HCl (for acidification) or NaOH (for basification) solution prior to experiments. The
double-walled cell containing the 9g/L NaCl solution was heated by the internal circulation of warm water
maintained at 38°C thanks to a Julabo F32 thermo-cryostat. The used potensiostat was a Versastat apparatus
(Princeton Applied Research - EGG), able to drive all the classical stationary tests. This device is, in addition,
equipped with an electronic card permitting to carry out Electrochemical Impedance Spectroscopy (EIS)
runs.
The experiments driven according to stationary methods were first the following of the open circuit potential (Eocp) for 2 hours and, second, the Tafel experiment. This later one was done by polarizing the working electrode from Eocp(2h) - 250mV up to Eocp(2h) + 250mV with a scan rate equal to 10mV/min. The obtained I=f(E) files were thereafter analysed according to the Tafel method, principally to reach precise values for the corrosion current density Icorr. The Ecorr potential as well as the two Tafel coefficients, the cathodic one (βc) and the anodic one (βa), were also specified.
In parallel EIS experiments were run by applying a potential varying from Ecorr - 10mV to Ecorr + 10mV as a sinusoidal function. The frequency varied from 10kHz down to 0.1Hz. This experiment aimed to the determination of a more accurate value of the transfer resistance Rt (by decoupling it from the electrolyte resistance Re) and to specify the double-layer capacity Cdl.
Results
The chemical compositions of all alloys were specified by EDS on the “MH” samples. Results show that the
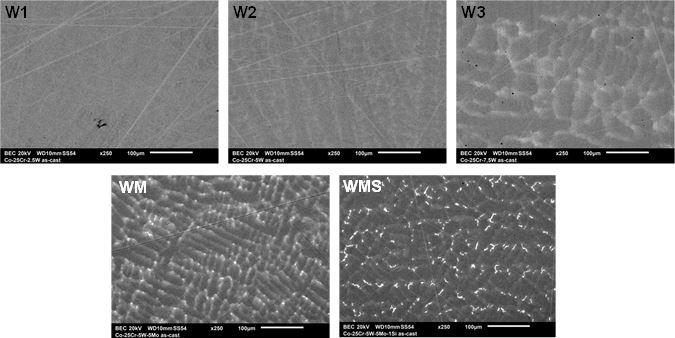
wished compositions (Table 1) were successfully reached. The as-cast alloys are either single-phased (W1 to
W3 and WM) or double-phased (WMS), as illustrated in Figure 4. The W1 alloy (Co-25Cr-2.5W, wt.%)
is seemingly totally homogeneous. This is almost also the case for the W2 alloy (Co-25Cr-5W) despite a
tendency to segregation of tungsten in the last solidified zones (dendrites periphery paler than the dendrites
core). This is more pronounced for the W3 alloy (Co-25Cr-7.5W) and particularly for the WM alloy (Co-
25Cr-5W-5Mo). In this later alloy both W and Mo obviously segregated together during solidification, this
inducing a locally high content in heavy elements in the dendrites boundaries. Despite these pronounced
chemical heterogeneities in their microstructures all the previous alloys were still single-phased. In contrast,
with addition of silicon, the WMS alloy (Co-25Cr-5W-5Mo-1Si) is to be considered as double-phased
because of the presence of small interdendritic precipitates rich in W and in Si, well separated from the
cobalt solid solution matrix.
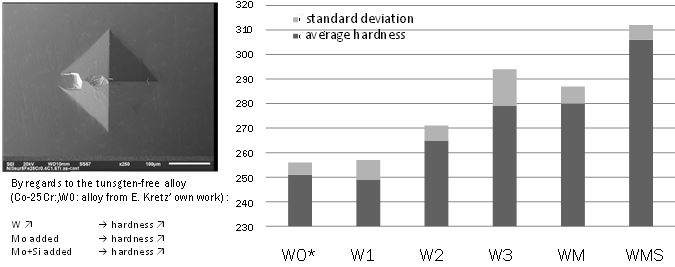
Up to five indentations were carried out on each “MH” sample. The average and standard deviation values
calculated from the measured hardness are graphically presented in Figure 5. In this figure a previous result obtained on a W-free binary alloy (noted “W0”, [16,17]) is added to better
specify the effect of the presence of tungsten. Introducing a so heavy element as tungsten in a cobaltchromium
alloy is expected to harden it by solid solution strengthening. In the W1 and W2 alloys the
greatest part of the W atoms is clearly present in solid solution. But this is only with the addition of 5
wt.%W that the hardening effect starts to be observed (by comparison to the reference W0 alloy added
for comparison). By increasing the W content to 7.5 wt.% (W3 alloy) a new increase in hardness is to be
noticed. Comparing the measured hardness of the “W2” and “WM” alloys allows seeing that the addition of
molybdenum is also favourable to the alloy’s hardness. Comparing the “WM” and “WMS” hardness results
leads to suggest that hardness takes also benefit from the interdendritic precipitation of a second phase rich
in W and Si, even if these particles ones are not numerous and rather small.
After 2 hours of immersion of the “EC” samples (electrodes) in the {37°C, 9g/L NaCl, pH7,4}-aqueous
artificial saliva Tafel experiments were carried out for each of the studied alloys. The obtained Tafel curves
are plotted in two groups. The first one allows the observation of the W content effect in the three ternary
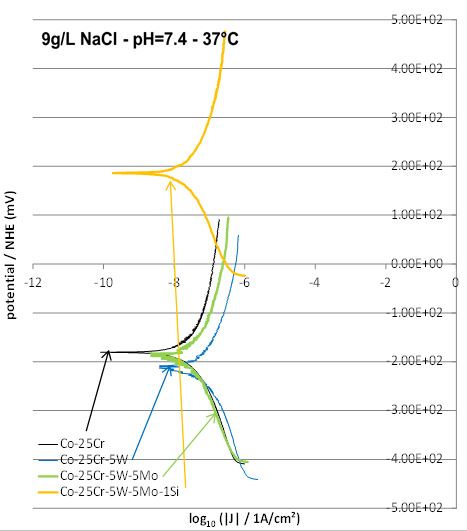
alloys W1, W2 and W3 (Figure 6, Table 2). The second one facilitates the study of the successive additions of
5 wt.%W, of 5wt.%Mo and of 1 wt.%Si (Figure 7, Table 3). In both cases the corresponding results obtained
for the binary W0 alloy are added.
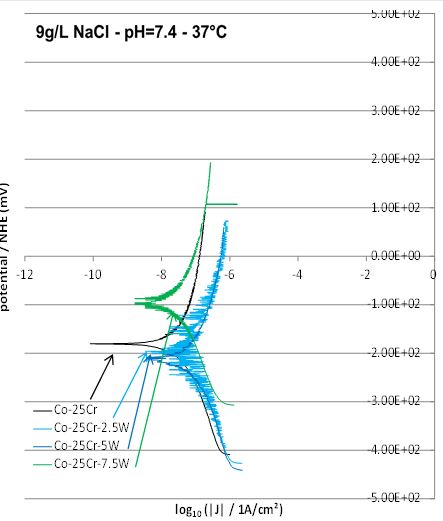
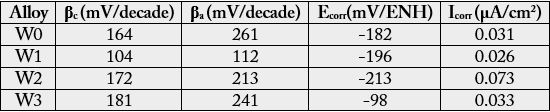
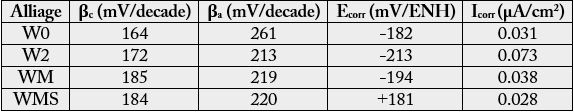
Concerning the effect of tungsten one can see that, after addition of 2.5 or 5wt.%W, the Tafel curve moves right (increased corrosion current density Icorr) and down (lower corrosion potential Ecorr). With the addition of 2.5 wt.%W more, the Tafel curve moves left (Icorr decreases) and up (Ecorr increases). The Tafel analysis confirms these qualitative observations (Table 2).
Concerning the successive additions of 5 wt.%W, 5 wt.%Mo and 1 wt.%Si, one logically finds again the slightly deleterious effect of tungsten on the corrosion behavior (by comparison to the W0 alloy). Adding Mo slightly improves a little this behaviour (small decrease in corrosion current and small increase in corrosion potential) while the addition of silicon minimizes again Icorr but has seemingly a huge effect on Ecorr which increases suddenly (Figure 7, Table 3).
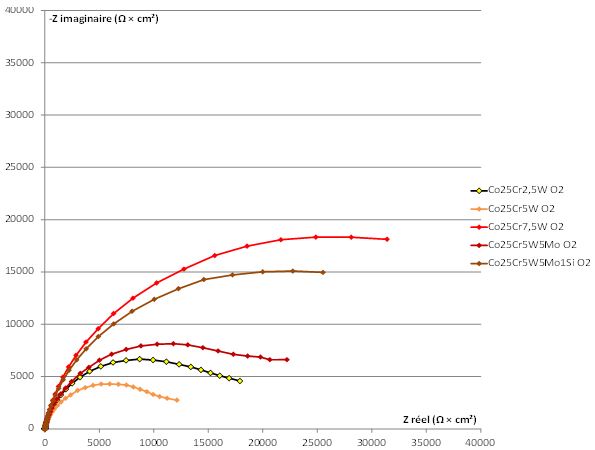
The EIS experiments were carried out for all the “EC” samples in the same electrolyte used previously for
simulating saliva. Each experiment was repeated five times for each alloy which allowed revealing a problem
of reproducibility: some discrepancies appeared for the EIS results obtained for a same alloy. This limits the
analysis of the results. Thereafter, the average result obtained for each alloy was considered, however, in order
to try exploiting the EIS experiments. As illustrated in Figure 8, all the obtained Nyquist diagrams (-Zim
= f(Zreal) with Zim: imaginary part of the complex impedance Z, Zreal: real part of Z) look like half a circle,
starting from the abscissa axis close to the ordinate one (very low modulus of Z, for the highest frequencies).
However, they never finished on the abscissa axis, even for the very low frequency of 0.1Hz. The diameter
of the half circles corresponding to the ternary alloys (Co-25Cr-xW) are the smallest and the almost half
of circle tended to go back to the abscissa axis. For the two more complex alloys, which contain Mo and/or
Si, the Nyquist diagrams are closer to quarter of circles than to half circles. The absolute value of Zim did not
really decrease when the frequency became very low. Their diameter are much greater, suggesting very high
values of the absolute values of the impedance (│Z(ω)│). The rather small diameter of the circle parts of
the ternary alloys (W1, W2 and W3) and the rather great diameter of the ones of the two {Mo and/or Si}-
containing alloys (WM and WMS) suggest high values of the polarisation resistance Rp = Rt + Re for the
later ones by comparison to the former ones. This is in good agreement with what was observed previously
with the stationary method since the higher the polarization resistance, the lower the corrosion current.
Because of the lack of reproducibility earlier evocated it was not tried to deduce an accurate value of the
transfer resistance Rt by subtracting the (very low) electrolyte resistance Re from the polarisation resistance
Rp (= diameter of the entire circle). However, one can say that Rt (and Rp) varies from about 10kΩ × cm2 (W1
to W3 alloys) to about 50kΩ × cm2 (WM and WMS alloys). In contrast, the double layer capacity stays close
to 2 × 10-5Farad/cm2 for all alloys.
Discussion
The presence and content of tungsten and silicon in the studied alloys had first indisputable effects on
the microstructures and on the hardness of these {Co-25Cr}-based alloys. This is clear that the addition
of tungsten and/or molybdenum was expected to make the microstructures more or less complex and to
strengthen the alloys mechanically. Obviously, adding 2.5 wt.% to 7.5 wt.% of tungsten induces more and
more segregation of the heavy element during solidification, allowing clearly distinguishing the dendritic
structure of these single-phase alloys. These W addition and enrichments favoured the presence of big atoms
in solid solution (where they substitute cobalt and chromium), responsible of a better resistance against
the dislocations’ movement when the stress applied by the indentation machine has become high enough
to drive the alloy in a plastic deformation. This strengthening effect is of course welcome for allowing the
metallic framework constituted of such alloy to resist mastication. The addition of molybdenum with the
same weight content as the tungsten one already present in the W2 alloy, as is to say the double quantity
of big atoms since the molar mass of W is twice the Mo one, exhibited a similar effect. W and Mo are
effectively two good solutions for reinforcing this type of alloys.
In another way, according to the results issued from the stationary methods and the EIS ones, all the studied alloys appeared to be highly resistant against corrosion in the (simple) artificial saliva considered here. As suggested by some free-potential follow-up (not showed here but rapid then slow increase in Eocp) and cyclic polarization runs (not showed here too, but absence of any anodic peak, and suspected transpassivation), one can guess that all alloys are more or less already passivated when the Tafel and EIS experiments are started. This may be notably the origin of the lack of reproducibility of the EIS results). Anyway, all these cobaltbased alloys behaved very well and are obviously very corrosion resistant, probably thanks to the 25wt.% of chromium that they all contain. However, one must also say that, concerning again the tungsten and/or molybdenum atoms added to the Co-25Cr base, it seems that W is unfortunately a little deleterious for the corrosion resistance of the alloys. In contrast Mo appears to be a useful element in this way. Silicon, although added in much lower weight content, it demonstrated a triple effect: 1/ more complex microstructure (the WMS alloy is double phased, unlike all the Si-free alloys of this study), 2/ increased hardness (and probably on the general mechanical properties including the flexural ones) and 3/ improved corrosion behaviour. The (Si, W) phase precipitated in the interdendritic boundaries, thanks to the well-known affinity of the two elements to form stoichiometric hard compounds, is probably WSi2, hypothesis which was unfortunately not checked because of the too small size of these particles for performing accurate spot analyses. Tungsten disilicide being very hard [18,19] this is not surprising that the strength of grain boundaries and of the interdendritic spaces was enhanced by the presence of these particles. Since it is an active element concerning oxidation and corrosion it may be also expected that Si localization in the interdendritic spaces may improve the corrosion behaviour of the alloy, what was effectively observed here.
One can summarize these results by saying that the cobalt-chromium alloys studied in this work showed high mechanical and corrosion resistances. These interesting properties can be numerically illustrated by the following numbers: hardness as high as 300Hv and corrosion current densities lower than 100nA/cm². However, the presence of minor elements demonstrated here their interest for some of them, for going further in quality, and the Co-25Cr-5W-5Mo-1Si alloy was here evidenced as the most interesting.
Now, deeper works for optimizing the contents in the selected elements for taking the highest benefits in term of mechanical resistance and of corrosion resistance, may be envisaged. In parallel, clinic tests may be carried out for observing the behaviour of parent alloys made of these alloys during real conditions of mastication and the reactivity of their emerging parts exposed to the buccal milieu in various situations (food, drinks, aeration…).
Conclusions
The mechanical properties of cobalt-chromium alloys appear to be high enough to endure the stresses
induced by mastication without risk of rupture. They are obviously also highly resistant against corrosion,
which allows avoiding ions release in mouth. They are thus able to be used as framework for strengthening
fixed partial denture. Among them this is the Co-25Cr-5W-5Mo-1Si alloy which demonstrated the most
interesting properties.
Acknowledgments
The authors thank Mathieu Lierre for the preparation of the solution as well as for his assistance.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!