Massimo Baudo1*, Mohamed Rahouma2*, Faiza Khan, M.3, Dina Ibrahim, A.4, Fatma Abou Elkasem5,
Mohamed Kamel2, Ihab Saad2, Maha Yehia5, Ihab Eldessouki6, Irbaz Hameed7 & Abdelrahman Mohamed2
1IRCCS Vita-Salute San Raffaele Hospital Via Olgettina, Italy
2Surgical Oncology Department, National Cancer Institute, Cairo University, Egypt
3Department of Surgery, Louisiana State University Health Sciences Center in New Orleans, USA
4Department of Medicine, Brown University, Providence, USA
5Medical Oncology Department, National Cancer Institute, Cairo University, Egypt
6Medical Oncology Department, University of Cincinnati Cancer Institute, Cincinnati, Ohio, USA
7Department of Surgery, Southeast University, Nanjing, China
*Equally contributed
*Correspondence to: Dr. Mohamed Rahouma, Surgical Oncology Department, National Cancer
Institute, Cairo University, Egypt, mhmdrahouma@gmail.com
Copyright © 2018 Dr. Mohamed Rahouma, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received: 11 December 2018
Published: 31 December 2018
Keywords: Thoracic Malignancy; Chemoradiotherapy; Lung Cancer
Cancer Incidence and Mortality
The World Health Organization (WHO) reports that cancer is the second leading cause of death globally
[1]. Focusing on thoracic cancers, there are distinct epidemiological pictures. Among all tumors, lung cancer has the highest incidence and mortality when both genders are taken into consideration together. However,
differences are present when each gender is observed individually. In males, lung cancer is the number one
cause of mortality and the second highest in incidence, while in females, it is the second most common
reason for mortality and the third highest in incidence. On the other hand, esophageal cancer ranks sixth
for overall mortality and tenth for overall incidence. It has a higher incidence (seventh) and mortality
(sixth) in males compared to females (thirteenth and tenth respectively) [2]. Invasive thymoma and thymic
carcinomas are rare tumors, consisting of about 1% of all malignancies, and the incidence and mortality rate
in males and females is very similar [3].
The appropriate treatment route that should be followed for every patient consists of a thorough
multidisciplinary evaluation characterized by an objective discussion of all available therapeutic and surgical
options with a full discussion of morbidity, mortality, and outcomes associated with each option. The
final decision takes into consideration many different factors including tumor histology, stage, location,
resectability, genetic mutations, and individual patient comorbidities and performance status (PS).
Therapeutic Options for Lung Cancer
For primary resectable early stage (stage I and II) non-small-cell lung cancer (NSCLC) the primary
treatment is surgery [4,5], as it provides the best long-term survival outcomes although SBRT Stereotactic
Body Radiation Therapy (SBRT) becomes highly competitive to surgery nowadays for Stage 1 <2cm [6].
Unfortunately, more than 70% of all NSCLC are diagnosed with advanced or metastatic disease (stage III or
IV) [7,8]. Resectable stage IIIA NSCLC are generally treated with a combination of surgery and adjuvant
chemotherapy [9]. Higher stage or unresectable NSCLC are treated with a combination of chemotherapies
(cisplatin or carboplatin with paclitaxel or pemetrexed in nonsquamous histology or gemcitabine) or a
combination of chemotherapy (cisplatin + etoposide or vinblastine or pemetrexed) and radiotherapy (the
optimal radiation schedule and dose remain topics of debate, but 1.5 Gy twice daily to a total of 45 Gy and
1.8-2.0 Gy daily to a total dose of 60-70 Gy are commonly used treatments [8,10]. The drug regimen with the
highest likelihood of benefit, with toxicity deemed acceptable to both the physician and the patient, should
be given as initial therapy for advanced lung cancer. Stage, weight loss, performance status (PS) and gender
predict survival [11]. Platinum-based chemotherapy prolongs survival [12], improves symptom control,
and yields superior quality of life compared to best supportive care. Histology of NSCLC is important for
selection of systemic therapy. Other therapeutic options for lung cancer include molecular targeted agents
and immunotherapy. In the first category, we find Erlotinib, Afatinib and Gefitinib targeting EGFR [13,14]
and Crizotinib, Alectinib and Ceritinib targeting ALK [15] and Avastin as angiogenesis inhibitor [16] The
second category includes immune checkpoint inhibitors, Pembrolizumab and Ipilimumab which are PD-1,
PDL-1, and CTLA-4 inhibitors, respectively [17,18].
Surgery is less effective in small-cell lung cancer (SCLC) and is only adopted for very early stages and followed
by chemotherapy. Most treatments use concurrent chemoradiotherapy or combination chemotherapy
(cisplatin or carboplatin + etoposide as 1st line and topotecan, irinotecan, docetaxel, nivolumab or ipilimumab
as 2nd line) [19].
Surgery in Lung Cancer
The past decades have been characterized by a rising interest in minimally invasive surgery, like videoassisted
thoracoscopic surgery (VATS) and robot-assisted thoracoscopic surgery (RATS), as an alternative
to traditional open thoracotomy. Figure 1 demonstrates the location of different incisions and port sites used
in the various surgical approaches available in the treatment of lung cancer.
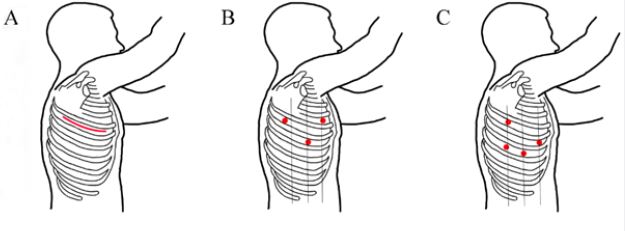
Figure 1: (A) Open lobectomy through 5th intercostal space (ICS) thoracotomy. (B) VATS lobectomy port sites: 4th
ICS at anterior axillary line, 5th ICS at tip of scapula, 6th ICS at midaxillary line. (C) RATS lobectomy port sites:
5th ICS at posterior axillary line, 6th ICS at anterior axillary line, 7th ICS at posterior and midaxillary line.
Several RCTs have investigated the outcomes of VATS versus thoracotomy. Kirby et al. highlighted that
VATS lobectomy is associated with fewer postoperative surgical complications [20]. The reduced operative
trauma of VATS pulmonary lobectomy resulted in reduced peri-operative changes in acute phase responses
[21]. The results of a study by Bendixen et al. demonstrated that VATS is associated with less postoperative
pain (p = 0.0012) and a better quality of life (p = 0.014) at 1-year follow-up [22]. Also seen were lower
perioperative mortality and complications, shorter length of hospital stay, and equal long-term survival
favoring VATS lobectomy [4]. No difference in 5-year survival (log-rank test, p = 0.74; generalized
Wilcoxon test, p = 0.91) was shown in the study by Sugi and colleagues [23]. However, perplexity about
the oncological equivalence of VATS compared to thoracotomy remains. Opposing results can be found in
literature suggesting either an increase in nodal upstaging with thoracotomy compared to VATS [24-26] or
no significant difference [20,27,28]. This variability may be explained by factors like the experience of the
institution and the learning curve as described in the study by Medbery et al. [24].
The more recent robotic version of minimally invasive surgery (RATS) demonstrates improvements in short
term outcomes like lower amounts of blood loss, mortality, morbidity, and shorter lengths of stay and chest
tube duration than traditional thoracotomy [22,29-32]. Though, the benefits of RATS over VATS are still
highly debated due to the lack of significant RCTs. Farivar et al. showed a decreased 30-day mortality and
postoperative blood transfusion rate after RATS versus VATS or thoracotomy [33].
Kent and colleagues concluded that RATS appears to be an appropriate alternative to VATS [34]. However,
Swanson et al. reported higher hospital costs and longer operating times without any differences in adverse
events [35]. Two recent meta-analyses concluded that RATS results in similar outcomes compared to VATS
[36,37]. RATS surely brings technological advantages over VATS such as three-dimensional imaging,
dexterity, seven degrees of freedom, lack of fulcrum effect and physiological tremors, scaled-down motions,
and ergonomic positioning. Nevertheless, high costs of equipment and maintenance and lack of tactile
sensation feedback are a downside of RATS [38,39]. Finally, the oncologic effectiveness of RATS still lacks
sufficient data to draw conclusions.
Therapeutic Options for Esophageal Cancer
Several randomized controlled trials (RCT) [33,40,41], in particular the Chemoradiotherapy for
Oesophageal Cancer Followed by Surgery Study (CROSS) [34], had confirmed that in locally advanced
esophageal cancer there is a survival benefit for using neoadjuvant chemoradiotherapy along with surgery
compared to surgery alone. Two recent meta-analyses have confirmed these results [35,42]. For early-stage
esophageal cancer, this advantage is still controversial. The Francophone de Cancérologie Digestive (FFCD)
9901 study that enrolled only stage I and II patients failed to demonstrate this survival benefit [33]. This
was in contrast to the CROSS study that also included early-stage tumors. A Phase III RCT showed that
the pathologic response and possibly outcomes as well are improved when neoadjuvant chemoradiotherapy
along with surgery is adopted compared to chemoradiation alone [43]. Although, no statistically significant
differences in survival have been observed in important studies comparing chemoradiotherapy with surgery
versus chemoradiotherapy alone [19,21,23]. A review by Swisher et al. that analyzed phase II/III RCTs
confirmed the superior results of combined chemoradiotherapy compared to chemotherapy alone [24]. The
3D conformal and Volumetric Arc Therapy (VMAT) radiation methods are adequate techniques to deliver
appropriate doses to the target. The two-plans with anterior- posterior, posterior- anterior and posterior
oblique fields had the most organ-sparing effect for lung and the liver. And three fields are to spare heart
and spinal cord. The total accepted dose is 50.4 Gy in 28 fractions [44].
Surgery in Esophageal Cancer
Esophagectomy, due to its highly invasive nature, still remains one of the most challenging surgeries as far
as operative morbidity and mortality are concerned [45]. Staging is a fundamental premise for a good longterm
surgical outcome. In fact, it identifies subgroups for neoadjuvant therapy and excludes patients with
metastatic or retrocarinal spread from surgery [46].
Over the years, various approaches were adopted from the initial open esophagectomy (OE) to more
advanced minimally invasive esophagectomy (MIE), including hybrid and robot-assisted, who’s aim is to
reduce the related complication of thoracotomy and laparotomy by performing the less invasive thoracoscopy
and laparoscopy [47,48]. Figure 2 demonstrates the location of different incisions and port sites used in
these various available surgical approaches. However, until now only one RCT, the TIME trial, has been
conducted to compare OE to MIE outcomes [47,48]. In this study, MIE was associated with significantly
fewer pulmonary infections at 2 weeks (p = 0.005) and in-hospital (p = 0.005), shorter operative time (p =
0.002) and hospital stay (p = 0.044), reduced intraoperative blood loss (p < 0.001) with equal 3-year overall- and disease-free survival [hazard ratio (HR): 0.883 (0.540-1.441) and HR: 0.691 (0.389-1.239),
respectively] [49]. Other recent studies showed equal or fewer morbidities, but higher reintervention rates of
MIE compared to OE [50-52]. Two recent meta-analysis confirmed these improved short-term outcomes
of MIE, but no difference in anastomotic leakage and the number of lymph nodes harvested was found
between the two techniques [53,54].

Figure 2: (A) Open esophagectomy: Ivor-Lewis (I+II); McKeown (I+II+III); Transhiatal (I+III). (B) VATS
esophagectomy port sites (thoracic phase): 4th and 9th ICS at posterior axillary line, 8th ICS at midaxillary line, 4th
ICS at anterior axillary line. (C) RAMIE port sites (thoracic phase): 5th and 7th ICS at midaxillary line, 8th ICS at
posterior axillary line, 9th ICS posterior to posterior axillary line.
A hybrid procedure (HMIE), consisting of thoracotomy and laparoscopy, was introduced to overcome the
challenging thoracoscopic phase that requires a long learning curve (estimated at 119 cases) before mastery
[55]. This approach is thought to be suited for low-volume centers with insufficient caseload to practice as
only the abdominal phase is performed minimally invasively. Nevertheless, the specific advantages of this
procedure are still not fully clear compared to OE or MIE due to lack of significant studies.
Another approach using robotic assistance (RAMIE) was introduced in order to help surgeons with this
demanding procedure. Augmented hand movements with additional degrees of freedom through scaling
and tremor filtration and the three-dimensional vision increases precision and accuracy [56]. RAMIE was
shown to be oncologically effective with R0 radical resections approaching 95% and providing adequate
lymphadenectomy with a low percentage of local recurrence seen at long-term follow up [57,58]. Other
advantages include better surgical access in reaching complex areas with conventional instruments [56,59].
On the other hand, RAMIE may present with some disadvantages such as longer operative time, higher
costs, and the lack of tactile feedback [60]. Few series with a significant number of patients, after the initial
small studies, have been published that compare RAMIE with OE or MIE [61,62].
Another form of minimally invasive approach is via endoscopy. Endoscopic mucosal resection (EMR) and
the novel endoscopic submucosal dissection (ESD) [63] are resection techniques developed to mainly target
early stages of esophageal and gastric cancers. In EMR, the idea is to apply suction towards the snare while
getting close to the lesion followed by cutting and cauterizing the surrounding mucosa, with the ability of
repeating in piecemeal fashion. ESD however allows wider superficial resection using the enbloc cauterization
technique while getting deep into the submucosa. Complications of resection approach generally include
bleeding, stricture and more importantly perforation, especially in the ESD technique [63].
Also as adjuvant therapy, Endoscopic ablation serves as other means in managing flat dysplastic and
neoplastic lesions, using techniques like Laser, Photodynamic Therapy (PDT), Argon Plasma Coagulation
(APC), Cryoablation and Radio Frequency Ablation (RFA) [64]. The National Comprehensive Cancer
Network (NCCN) guidelines, proposed adopting the endoscopic resection techniques, with possibly adding
ablation, as an alternative for early TNM staging of esophageal cancer as well as medically unfit patients for
surgery [65].
Therapeutic Options for Thymoma
Due to its rarity, there are very few RCTs that are available for planning for this disease. Therefore, the
analysis of the few published trials and studies is the only available approach to build the treatment
guidelines [66]. Thymoma staging adopts the Masaoka system with little modification from the original
paper [67]. Masaoka stage I and II thymoma is resected by surgery. For stage I surgery is performed without
neoadjuvant or adjuvant chemotherapy, while in stage II adjuvant chemotherapy may be considered for
high risk patients. For a resectable stage III thymoma, surgery in combination with neoadjuvant or adjuvant
chemotherapy is considered. For a non-resectable stage III thymoma chemotherapy with or without
radiation therapy is recommended with a median dose of radiotherapy of 50 Gy. In stage IVA thymoma,
pleural dissemination determines the therapy: when it’s not extensive, surgery with or without neoadjuvant
chemotherapy is recommended; while, when it’s extensive, chemotherapy is recommended. Finally, for
stage IVB thymoma, chemotherapy is generally recommended [68-70]. The treatment regimens includes
CAP (Cisplatin, Doxorubicin, Cyclophosphamide), CAP with prednisone, PE( Cisplatin, Etoposide), VIP
(Etoposide, Ifosfamide, Cisplatin) and Carboplatin-Paclitaxel [71].
Surgery in Thymoma
Sternotomy is considered the gold standard surgical approach to the thymus [72]. The surgical treatment
objective is to obtain a complete resection of the tumor and the open approach provides complete access and
visualization of the mediastinum for optimal results. In the past years, VATS thymectomy gained increasing
popularity because it was associated with reduced intraoperative blood loss, less postoperative pain, fewer
post-operative pneumonias, shorter length of hospital stay, and at least similar survival and recurrence rates
compared to the open approach [72-79]. Large tumors do not always prohibit minimally invasive approaches,
but caution must be taken in certain cases when tumors are severely adherent to or invade into vital organs
[80]. The robot-assisted approach is believed to improve minimally invasive thymectomy compared to VATS
[81-83]. Figure 3 demonstrates the location of different incisions and port sites used in all of the surgical
approaches mentioned.
Challenging anatomical layouts of the thymic gland increase the difficulty of VATS. Adopting a robotic
approach can minimize these problems and this might translate into superior resections. Preliminary
oncologic outcomes and survival for the robotic technique are promising, but the number of significant
studies and the number of patients included in these studies are still lacking to better define the role of
robotic thymectomy in the surgical treatment of thymoma [84-93].

Figure 3: (A) Open thymectomy: transcervical approach (blue line); transsternal apporach (red line). (B) VATS
thyrodectomy port sites: 4th ICS at posterior axillary line, 5th ICS at anterior axillary line, 6th ICS at midaxillary
line. (C) Robotic thyrodectomy port sites: 3rd ICS at anterior axillary line, 5th ICS at midaxillary and midclavicular
line.
Conclusions
In order to provide the best quality of care, treatment for thoracic cancers should be performed by an
experienced multidisciplinary team. Surgery remains the main treatment for thymomas and early-stage
NSCLC, and it is a fundamental therapeutic option for the combination therapy of esophageal cancer.
Minimally invasive techniques, like thoracoscopic and robot-assisted approaches, are increasingly used and
show promising results. Though more randomized studies are warranted to better clarify the efficacy of these
minimally invasive techniques especially the robotic technique.
Bibliography
- Ferlay, J., Soerjomataram, I., Dikshit, R., et al. (2015). Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012: Globocan 2012. Int. J. Cancer., 136(5), E359-E386.
- Global Burden of Disease Cancer Collaboration, Fitzmaurice, C., Akinyemiju, T. F., et al. (2018). Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol., 4(11), 1553-1568.
- Kim, E. & Thomas, C. R. (2015). Conditional survival of malignant thymoma using national population-based surveillance, epidemiology, and end results (SEER) registry (1973-2011). J. Thorac. Oncol., 10(4), 701-707.
- Howington, J. A., Blum, M. G., Chang, A. C., et al. (2013). Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest, 143(5 Suppl), e278S-e313S.
- Altorki, N. K., Kamel, M. K., Narula, N., et al. (2016). Anatomical Segmentectomy and Wedge Resections Are Associated with Comparable Outcomes for Patients with Small cT1N0 Non-Small Cell Lung Cancer. J. Thorac. Oncol., 11(11), 1984-1992.
- Paul, S., Lee, P. C., Mao, J., et al. (2016). Long term survival with stereotactic ablative radiotherapy (SABR) versus thoracoscopic sublobar lung resection in elderly people: national population based study with propensity matched comparative analysis. Bmj., 354(i3570).
- Travis, W. D., Brambilla, E. & Riely, G. J. (2013). New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J. Clin. Oncol., 31(8), 992-1001.
- Shokralla, H. A. & Rahouma, M. (2016). Prognostic Clinico-Pathological Features of 99 Cases Advanced Non-Small Cell Lung Cancer-Egyptian National Cancer Institute. Adv. Lung Cancer, 4(3), 29.
- Scagliotti, G. V., Fossati, R., Torri, V., et al. (2003). Randomized study of adjuvant chemotherapy for completely resected stage I, II, or IIIA non-small-cell Lung cancer. J. Natl. Cancer Inst., 95(19), 1453-1461.
- Stinchcombe, T. E. & Gore, E. M. (2010). Limited-stage small cell lung cancer: current chemoradiotherapy treatment paradigms. The Oncologist, 15(2), 187-195.
- Liu, L., Shi, M., Wang, Z., et al. (2018). A molecular and staging model predicts survival in patients with resected non-small cell lung cancer. BMC Cancer., 18(1), 966.
- Chen, L. K., Liang, Y., Yang, Q. Y., et al. (2012). Triplet platinum-based combination sequential chemotherapy improves survival outcome and quality of life of advanced non-small cell lung cancer patients. Asian Pac. J. Cancer Prev., 13(5), 1863-1867.
- Shepherd, F. A., Rodrigues Pereira, J., Ciuleanu, T., et al. (2005). Erlotinib in previously treated non-small-cell lung cancer. N. Engl. J. Med., 353(2), 123-132.
- Stiles, B. M., Nasar, A., Hussein, M. K., et al. (2016). Routine molecular testing of resected early-stage lung adenocarcinoma with targeted next-generation sequencing demonstrates a high rate of actionable mutations. J. Thorac. Oncol., 11(2), S44-S45.
- Shaw, A. T., Yeap, B. Y., Solomon, B. J., et al. (2011). Effect of crizotinib on overall survival in patients with advanced non-small-cell lung cancer harbouring ALK gene rearrangement: a retrospective analysis. Lancet Oncol., 12(11), 1004-1012.
- Ellis, P. M. & Al-Saleh, K. (2012). Multitargeted anti-angiogenic agents and NSCLC: clinical update and future directions. Crit. Rev. Oncol. Hematol., 84(1), 47-58.
- Thomas, A. & Hassan, R. (2012). Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol., 13(7), e301-310.
- Rahouma, M., Baudo, M., Yahia, M., et al. (2017). PS02. 10 Pneumonitis as a Complication of anti-PD/PDL1 Immunotherapy: A Meta-Analysis of Randomized Clinical Trials: Topic: Medical Oncology. J. Thorac. Oncol., 12(11), S1567-S1568.
- Früh, M., De Ruysscher, D., Popat, S., et al. (2013). Small-cell lung cancer (SCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol., 24(Suppl 6), vi99-105.
- D’Amico, T. A., Niland, J., Mamet, R., et al. (2011). Efficacy of mediastinal lymph node dissection during lobectomy for lung cancer by thoracoscopy and thoracotomy. Ann. Thorac. Surg., 92(1), 226-231, discussion 231-232.
- Craig, S. R., Leaver, H. A., Yap, P. L., et al. (2001). Acute phase responses following minimal access and conventional thoracic surgery. Eur. J. Cardio-Thorac. Surg., 20(3), 455-463.
- Cerfolio, R. J., Bryant, A. S., Skylizard, L. & Minnich, D. J. (2011). Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J. Thorac. Cardiovasc. Surg., 142(4), 740-746.
- Sugi, K., Kaneda, Y. & Esato, K. (2000). Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J. Surg., 24(1), 27-30, discussion 30-31.
- Medbery, R. L., Gillespie, T. W., Liu, Y., et al. (2016). Nodal Upstaging Is More Common with Thoracotomy than with VATS During Lobectomy for Early-Stage Lung Cancer: An Analysis from the National Cancer Data Base. J. Thorac. Oncol., 11(2), 222-233.
- Licht, P. B., Jørgensen, O. D., Ladegaard, L. & Jakobsen, E. (2013). A national study of nodal upstaging after thoracoscopic versus open lobectomy for clinical stage I lung cancer. Ann. Thorac. Surg., 96(3), 943-949, discussion 949-950.
- Boffa, D. J., Kosinski, A. S., Paul, S., et al. (2012). Lymph node evaluation by open or video-assisted approaches in 11,500 anatomic lung cancer resections. Ann. Thorac. Surg., 94(2) 347-353, discussion 353.
- Yang, C. J., Kumar, A., Klapper, J. A., et al. (2017). A National Analysis of Long-term Survival Following Thoracoscopic Versus Open Lobectomy for Stage I Non-small-cell Lung Cancer. Ann. Surg., 269(1), 163-171.
- Yang, C. J., Sun, Z., Speicher, P. J., et al. (2016). Use and Outcomes of Minimally Invasive Lobectomy for Stage I Non-Small Cell Lung Cancer in the National Cancer Data Base. Ann. Thorac. Surg., 101(3), 1037-1042.
- Adams, R. D., Bolton, W. D., Stephenson, J. E., et al. (2014). Initial multicenter community robotic lobectomy experience: comparisons to a national database. Ann. Thorac. Surg., 97(6), 1893-1898, discussion 1899-1900.
- Farivar, A. S., Cerfolio, R. J., Vallières, E., et al. (2014). Comparing robotic lung resection with thoracotomy and video-assisted thoracoscopic surgery cases entered into the Society of Thoracic Surgeons database. Innov. Phila., 9(1), 10-15.
- Kent, M., Wang, T., Whyte, R., et al. (2014). Open, video-assisted thoracic surgery, and robotic lobectomy: review of a national database. Ann. Thorac. Surg., 97(1), 236-242, discussion 242-244.
- Swanson, S. J., Miller, D. L., McKenna, R. J., et al. (2014). Comparing robot-assisted thoracic surgical lobectomy with conventional video-assisted thoracic surgical lobectomy and wedge resection: results from a multihospital database (Premier). J. Thorac. Cardiovasc. Surg., 147(3), 929-937.
- Mariette, C., Dahan, L., Mornex, F., et al. (2014). Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J. Clin. Oncol., 32(23), 2416-2422.
- van Hagen, P., Hulshof, M. C., van Lanschot, J. J., et al. (2012). Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med., 366(22), 2074-2084.
- Sjoquist, K. M., Burmeister, B. H., Smithers, B. M., et al. (2011). Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol., 12(7), 681-692.
- Ye, X., Xie, L., Chen, G., et al. (2015). Robotic thoracic surgery versus video-assisted thoracic surgery for lung cancer: a meta-analysis. Interact. Cardiovasc. Thorac. Surg., 21(4), 409-414.
- Wei, S., Chen, M., Chen, N. & Liu, L. (2017). Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J. Surg. Oncol., 15(1), 98.
- Melfi, F. M. A. & Mussi, A. (2008). Robotically assisted lobectomy: learning curve and complications. Thorac. Surg. Clin., 18(3), 289-295, vi-vii.
- Wei, B. & D’Amico, T. A. (2014). Thoracoscopic versus robotic approaches: advantages and disadvantages. Thorac. Surg. Clin., 24(2), 177-188, vi.
- Tepper, J., Krasna, M. J., Niedzwiecki, D., et al. (2008). Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J. Clin. Oncol., 26(7), 1086-1092.
- Oppedijk, V., van der Gaast, A., van Lanschot, J. J. B., et al. (2014). Patterns of recurrence after surgery alone versus preoperative chemoradiotherapy and surgery in the CROSS trials. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol., 32(5), 385-391.
- Wang, D. B., Zhang, X., Han, H. L., et al. (2012). Neoadjuvant chemoradiotherapy could improve survival outcomes for esophageal carcinoma: a meta-analysis. Dig. Dis. Sci., 57(12), 3226-3233.
- Stahl, M., Walz, M. K., Stuschke, M., et al. (2009). Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J. Clin. Oncol., 27(6), 851-856.
- Fawaz, Z. S., Kazandjian, S., Tsui, J. M., et al. (2018). What Is the Optimal Radiation Technique for Esophageal Cancer? A Dosimetric Comparison of Four Techniques. Cureus, 10(7), e2985.
- Steyerberg, E. W., Neville, B. A., Koppert, L. B., et al. (2006). Surgical mortality in patients with esophageal cancer: development and validation of a simple risk score. J. Clin. Oncol., 24(26), 4277-4284.
- Swisher, S. G., Hofstetter, W., Komaki, R., et al. (2010). Improved long-term outcome with chemoradiotherapy strategies in esophageal cancer. Ann. Thorac. Surg., 90(3), 892-898, discussion 898-899.
- Luketich, J. D., Alvelo-Rivera, M., Buenaventura, P. O., et al. (2003). Minimally invasive esophagectomy: outcomes in 222 patients. Ann. Surg., 238(4), 486-494, discussion 494-495.
- Smithers, B. M., Gotley, D. C., Martin, I. & Thomas, J. M. (2007). Comparison of the outcomes between open and minimally invasive esophagectomy. Ann. Surg., 245(2), 232-240.
- Straatman, J., van der Wielen, N., Cuesta, M. A., et al. (2017). Minimally Invasive Versus Open Esophageal Resection: Three-year Follow-up of the Previously Reported Randomized Controlled Trial the TIME Trial. Ann. Surg., 266(2), 232-236.
- Sihag, S., Kosinski, A. S., Gaissert, H. A., et al. (2016). Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Comparison of Early Surgical Outcomes From The Society of Thoracic Surgeons National Database. Ann. Thorac. Surg., 101(4), 1281-1288, discussion 1288-1289.
- Seesing, M. F. J., Gisbertz, S. S., Goense, L., et al. (2017). A Propensity Score Matched Analysis of Open Versus Minimally Invasive Transthoracic Esophagectomy in the Netherlands. Ann. Surg., 266(5), 839-846.
- Takeuchi, H., Miyata, H., Ozawa, S., et al. (2017). Comparison of Short-Term Outcomes Between Open and Minimally Invasive Esophagectomy for Esophageal Cancer Using a Nationwide Database in Japan. Ann. Surg. Oncol.. 24(7), 1821-1827.
- Wang, B., Zuo, Z., Chen, H., et al. (2017). The comparison of thoracoscopic-laparoscopic esophagectomy and open esophagectomy: A meta-analysis. Indian J. Cancer, 54(1), 115-119.
- Yibulayin, W., Abulizi, S., Lv, H. & Sun, W. (2016). Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: a meta-analysis. World J. Surg. Oncol., 14(1), 304.
- van Workum, F., Stenstra, M. H. B. C., Berkelmans, G. H. K., et al. (2019). Learning Curve and Associated Morbidity of Minimally Invasive Esophagectomy: A Retrospective Multicenter Study. Ann. Surg., 269(1), 88-94.
- Ruurda, J. P., van der Sluis, P. C., van der Horst, S. & van Hilllegersberg, R. (2015). Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J. Surg. Oncol., 112(3), 257-265.
- van Hillegersberg, R., Boone, J., Draaisma, W. A., et al. (2006). First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg. Endosc., 20(9), 1435-1439.
- van der Sluis PC, Ruurda JP, Verhage, R. J. J., et al. (2014). Oncologic Long-Term Results of Robot-Assisted Minimally Invasive Thoraco-Laparoscopic Esophagectomy with Two-Field Lymphadenectomy for Esophageal Cancer. Ann. Surg. Oncol., 22(Suppl 3), S1350-1356.
- van der Horst, S., Weijs, T. J., Ruurda, J. P., et al. (2017). Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy for esophageal cancer in the upper mediastinum. J. Thorac. Dis., 9(Suppl 8), S834-S842.
- He, H., Wu, Q., Wang, Z., et al. (2018). Short-term outcomes of robot-assisted minimally invasive esophagectomy for esophageal cancer: a propensity score matched analysis. J. Cardiothorac. Surg., 13(1), 52.
- Weksler, B. & Sullivan, J. L. (2017). Survival After Esophagectomy: A Propensity-Matched Study of Different Surgical Approaches. Ann. Thorac. Surg., 104(4), 1138-1146.
- Yerokun, B. A., Sun, Z., Yang, C. F. J., et al. (2016). Minimally Invasive Versus Open Esophagectomy for Esophageal Cancer: A Population-Based Analysis. Ann. Thorac. Surg., 102(2), 416-423.
- Guo, H. M., Zhang, X. Q., Chen, M., et al. (2014). Endoscopic submucosal dissection vs endoscopic mucosal resection for superficial esophageal cancer. World J. Gastroenterol., 20(18), 5540-5547.
- Balmadrid, B., Hwang, J. H. (2015). Endoscopic resection of gastric and esophageal cancer. Gastroenterol. Rep., 3(4), 330-338.
- Ajani, J. A., Barthel, J. S., Bentrem, D. J., et al. (2011). Esophageal and esophagogastric junction cancers. J. Natl. Compr. Cancer Netw., 9(8), 830-887.
- Fujii, Y. (2011). Published guidelines for management of thymoma. Thorac. Surg. Clin., 21(1), 125-129.
- Masaoka, A., Monden, Y., Nakahara, K. & Tanioka, T. (1981). Follow-up study of thymomas with special reference to their clinical stages. Cancer, 48(11), 2485-2492.
- Falkson, C. B., Bezjak, A., Darling, G., et al. (2009). The management of thymoma: a systematic review and practice guideline. J. Thorac. Oncol., 4(7), 911-919.
- PDQ Adult Treatment Editorial Board. Thymoma and Thymic Carcinoma Treatment (PDQ®): Health Professional Version. PDQ Cancer Inf. Summ., Bethesda (MD): National Cancer Institute (US), 2002.
- Kamel, M. K., Stiles, B. M., Ghaly, G., et al. (2016). Predictors of pleural implants in patients with thymic tumors. Ann. Thorac. Surg., 102(5), 1647-1652.
- NCCN - Evidence-Based Cancer Guidelines, Oncology Drug Compendium, Oncology Continuing Medical Education.
- Kondo, K. (2014). Therapy for thymic epithelial tumors. Gen. Thorac. Cardiovasc. Surg., 62(8), 468-474.
- Wagner, A. J., Cortes, R. A., Strober, J., et al. (2006). Long-term follow-up after thymectomy for myasthenia gravis: thoracoscopic vs open. J. Pediatr. Surg., 41(1), 50-54.
- Hiratsuka, M., Iwasaki, A., Shirakusa, T., et al. (2006). Role of video-assisted thoracic surgery for the treatment of myasthenia gravis: extended thymectomy by median sternotomy versus the thoracoscopic approach with sternal lifting. Int. Surg., 91(1), 44-51.
- Toker, A., Eroglu, O., Ziyade, S., et al. (2005). Comparison of early postoperative results of thymectomy: partial sternotomy vs. videothoracoscopy. Thorac. Cardiovasc. Surg., 53(2), 110-113.
- Lin, T. S., Tzao, C., Lee, S. C., et al. (2005). Comparison between video-assisted thoracoscopic thymectomy and transternal thymectomy for myasthenia gravis (analysis of 82 cases). Int. Surg., 90(1), 36-41.
- Ng, C. S. H., Wan, I. Y. P. & Yim, A. P. C. (2010). Video-assisted thoracic surgery thymectomy: the better approach. Ann. Thorac. Surg., 89(6), S2135-2141.
- Xie, A., Tjahjono, R., Phan, K. & Yan, T. D. (2015). Video-assisted thoracoscopic surgery versus open thymectomy for thymoma: a systematic review. Ann. Cardiothorac. Surg., 4(6), 495-508.
- Kamel, M. K., Rahouma, M., Stiles, B. M., et al. (2017). Robotic Thymectomy: Learning Curve and Associated Perioperative Outcomes. J. Laparoendosc. Adv. Surg. Tech., 27(7), 685-690.
- Limmer, K. K. & Kernstine, K. H. (2011). Minimally invasive and robotic-assisted thymus resection. Thorac. Surg. Clin., 21(1), 69-83.
- Yoshino, I., Hashizume, M., Shimada, M., et al. (2001). Thoracoscopic thymomectomy with the da Vinci computer-enhanced surgical system. J. Thorac. Cardiovasc. Surg., 122(4), 783-785.
- Rückert, J. C., Swierzy, M., Ismail, M. (2011). Comparison of robotic and nonrobotic thoracoscopic thymectomy: a cohort study. J. Thorac. Cardiovasc. Surg., 141(3), 673-677.
- Kneuertz, P. J., Kamel, M. K., Stiles, B. M., et al. (2017). Robotic thymectomy is feasible for large thymomas: a propensity-matched comparison. Ann. Thorac. Surg., 104(5), 1673-1678.
- Schneiter, D., Tomaszek, S., Kestenholz, P., et al. (2013). Minimally invasive resection of thymomas with the da Vinci® Surgical System. Eur. J. Cardio-Thorac. Surg., 43(2), 288-292.
- Rückert, J. C., Ismail, M., Swierzy, M., et al. (2008). Thoracoscopic thymectomy with the da Vinci robotic system for myasthenia gravis. Ann. N. Y. Acad. Sci., 1132, 329-335.
- Marulli, G., Schiavon, M., Perissinotto, E., et al. (2013). Surgical and neurologic outcomes after robotic thymectomy in 100 consecutive patients with myasthenia gravis. J. Thorac. Cardiovasc. Surg., 145(3), 730-735.
- Melfi, F., Fanucchi, O., Davini, F., et al. (2012). Ten-year experience of mediastinal robotic surgery in a single referral centre. Eur. J. Cardio-Thorac. Surg., 41(4), 847-851.
- Augustin, F., Schmid, T., Sieb, M., et al. (2008). Video-assisted thoracoscopic surgery versus robotic-assisted thoracoscopic surgery thymectomy. Ann. Thorac. Surg., 85(2), S768-771.
- Castle, S. L. & Kernstine, K. H. (2008). Robotic-assisted thymectomy. Semin. Thorac. Cardiovasc. Surg., 20(4), 326-331.
- Goldstein, S. D. & Yang, S. C. (2010). Assessment of robotic thymectomy using the Myasthenia Gravis Foundation of America Guidelines. Ann. Thorac. Surg., 89(4), 1080-1085.
- Stahl, M., Stuschke, M., Lehmann, N., et al. (2005). Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J. Clin. Oncol., 23(10), 2310-2317.
- Bedenne, L., Michel, P., Bouché, O., et al. (2007). Chemoradiation followed by surgery compared with chemoradiation alone in squamous cancer of the esophagus: FFCD 9102. J. Clin. Oncol., 25(10), 1160-1168.
- Bonnetain, F., Bouché, O., Michel, P., et al. (2006). A comparative longitudinal quality of life study using the Spitzer quality of life index in a randomized multicenter phase III trial (FFCD 9102): chemoradiation followed by surgery compared with chemoradiation alone in locally advanced squamous resectable thoracic esophageal cancer. Ann. Oncol., 17(5), 827-834.



