Biography
Interests
Saranya Ramalingam Singaravelu
Department of Biotechnology, Karpagam Academy of Higher Education, Coimbatore, Tamilnadu, India
*Correspondence to: Dr. Saranya Ramalingam Singaravelu, Department of Biotechnology, Karpagam Academy of Higher Education, Coimbatore, Tamilnadu, India.
Copyright © 2018 Dr. Saranya Ramalingam Singaravelu. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
The present study was to evaluate the chewers has the morphological and pathological changes
among the chewers.
We analyzed the cytomorphological and the genotoxicity has been seen in chewers of exposed
groups in building workers 30 building construction workers and number of age and sex matched
controls. For each individual, 2000 exfoliated buccal cells were analyzed.
The results observed in the present study were, a clear proportional increase in CD was shown in
those aged 25-45, a decrease in ND chewers users and a steady increase in N/C ratio from control
individuals to chewers users (P<0.05).
Our results specify that building construction workers exposed to PAHs may associate with
genotoxic effects. Nonetheless it is important to create better awareness of occupational hazards
among workers to promote occupational safety.
Introduction
The carcinogenic potential of petroleum hydrocarbons was examined in a critical review of world literature
in the period 1960-1978. Although the carcinogenic potential of some samples of petroleum and other
fossil-fuel material tested in experimental animals could be associated with the presence of benzo[a]pyrene
(BaP), others without benzo[a]pyrene were also carcinogenic [1].
In an literature survey, an attempt has been made to study the contribution of bulk deposition PAHs into various other environmental compartments including soil, street dust, river water and sediment. Polycyclic aromatic hydrocarbon compounds are formed and released into the environment through both natural and anthropogenic sources [2].
Natural sources include volcanoes and forest fires. Anthropogenic sources include: combustion of fossil fuels, including motor vehicle emission and power generation wood burning, municipal and industrial waste incineration, coal tar, coke, asphalt, crude oil, creosote, asphalt roads, roofing [3].
Occupational and environmental exposures mostly represent mixtures of genotoxic agents, whereas the specificity of biomarker measurements varies widely. Exploration of correlations among biomarkers contributes to the further progress of molecular cancer epidemiology and to the selection of the optimal biomarkers for the investigation of human exposure to carcinogens [4].
Epidemiological studies found an increased risk of cancers in occupations exposed to traffic air pollution. PM2.5 are toxic and can enter into the respiratory tract and circulatory system. PM2.5 can adsorb various substances, such as polycyclic aromatic hydrocarbons (PAHs) nitro-PAHs. The present study was carried out with 15 Reboucas tunnel (Rio de) Samples of buccal mucosa cells and peripheral blood were evaluated using micronucleus (MN) assay. Higher concentrations of 1-OHP (16.47 μmol/molcreatinine were also detected in the exposure group [5].
The comet assay is the method of choice for measuring DNA damage, of various sorts, in human cells such as lymphocytes obtained in the course of population-based studies of environmental and occupational exposure to different genotoxic agents, including radiation, chemicals and oxidative stress. In terms of simplicity, cost, small amount of material required, sensitivity and reliability, the comet assay in its various modifications has few serious competitors. When standardized and validated, the comet assay can provide invaluable information in the areas of hazard identification and risk assessment of environmental and occupational exposure, diseases linked with oxidative stress (e.g. diabetes and cardiovascular disease), nutrition, monitoring the effectiveness of medical treatment and investigating individual variation in response to DNA damage that may reflect genetic or environmental influences [6]. The information obtained could lead to individual advice on lifestyle changes to promote health and especially on relative risks of genotoxic exposure to environmental pollution [7].
The present work aims at integrating genotoxicity damage induced by workplace stress-related effects observed in workers collected during working period from an impacted estuary. Distinct patterns were observed in cells exposed to crude mixtures of sediment contaminants from the urban/industrial area comparatively to the ones from the controls, with respect to oxidative DNA damage. This observation suggests that metals and unknown toxicants more hydrophilic than polycyclic aromatic hydrocarbons may be important causative agents, especially in samples from the building construction workers, where oxidative DNA damage was the most significant [8].
Urinary 1-hydroxypyrene (1-OHP) was selected as a biomarker of polycyclic aromatic hydrocarbons (PAHs) to explore the accumulation level in the bodies of workers at rubber smoke sheet factories in southern Thailand [9].
Material and Methods
The total study population was 60 individuals; it comprised 30 building workers and 30 controls. All of
the study subjects were men and aged from 25 to 45 years. The workers had varying durations of exposure
(5-15years).They were further branched into smokers and non-smokers, chewers along with smokers and
chewers alone. The control group was selected from the general population with no history of occupational
exposure to PAHs or any known physical or chemical agent in the workplace, but belongs to the same age
group and socio-economic status as the building workers. The study was conducted in accordance with the
principles foe human experience as defined by the Helsinki declaration, 1964.
The buccal sample was collected from each subject after the work shift. Workers were asked to rinse their mouth with distilled water. The buccal cells were collected using a toothpick by scrapping the inside cheek of the mouth. The toothpick was agitated in 1ml of saline and the suspension was centrifuged at 1500rpm for 10minutes. The cell pellet was resuspended in 1ml saline. The obtained cell pellet was used for cytomorphometric analysis, micronuclei analysis and comet assay.
Urine samples were collected in polyethylene bottles (which had been washed with 0.2% HNO3). At the end of the work shift and they were kept cold (±4°C) during collection and with 20% glycerol (volume for volume) minimize cells loss due to lysis after freezing (-20°C).
Results
The present study focuses on 60 individuals, 30 controls and 30 building construction workers. the study
subjects were chosen from different building construction workers in and around Coimbatore city along in
the age and sex matched controls.
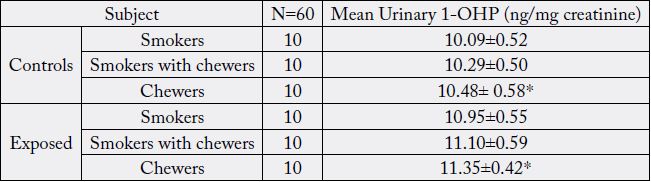
Table 1: Summarizes the general characteristics of study population, which shows the grouping of both control and building construction workers based on smoking, smoking with chewers and chewers habit.
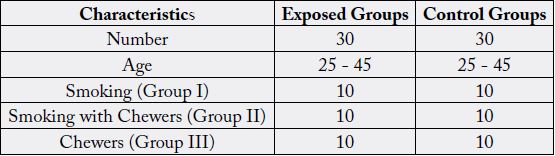
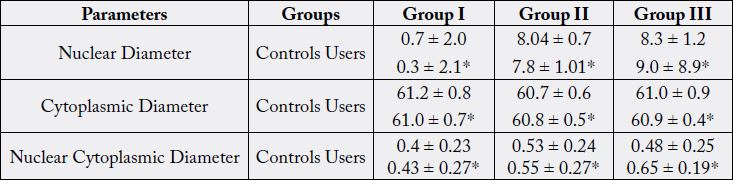
All values are expressed as mean ± SD.*P < 0.05
The buccal mucosa of healthy volunteers with smoking habit alone smoking with chewing habit and chewing alone showed a significant variation in the size of mean ND and CD when compared with the respective controls. The results showed that mean ND in buccal mucosa was noticeably elevated (p<0.05) in users group (0.45±0.23, 0.53±0.24, 0.48 ± 0.25μm) than in the control group (0.7±2.0, 8.04 ± 0.7, 8.3 ± 1.2μm), and mean CD in buccal mucosa was markedly lower (p<0.05) in user group (61.0 ± 0.7, 60.8 ± 0.5, 60.9 ± 0.4μm) than in the control group (61.2 ± 0.8, 60.7 ± 0.6, 61.0 ± 0.9μm). In addition, mean N/C ratio in users group (0.43 ± 0.27, 0.55 ± 0.27, 0.65 ± 0.19) was apparently higher than in the control group (0.43 ± 0.27, 0.55 ± 0.27, 0.48 ± 0.25) (p<0.05).
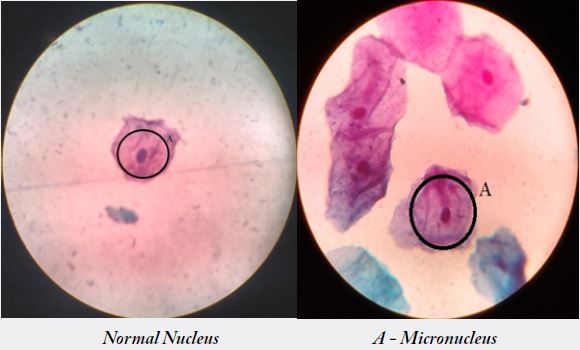
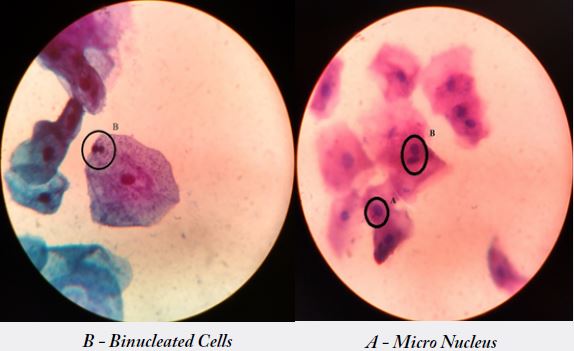
In the present study, increased occurrence of abnormal nucleus and cytoplasm ratio was noticed in the
exfoliated cells of the chewers users. Increase in age and extent of chewers use showed a significantly
higher frequency of every investigated cytological changes. This finding is positively associated with oral
carcinogenesis and support earlier investigations that disclose statistically significant decline in mean
cytoplasmic area of cells taken from normal buccal mucosa of tobacco chewers [10,11]. The buccal mucosa
collected from 25-45 years of age group chewer’s users also showed a significant result when compared to
that of controls.
Increase in nuclear size is an indicator of cellular damage in tobacco users. Decreased cellular turnover as a result of prolonged chewers use following ageing would result in more number of mature cells with large nuclei in the smear [12]. This also accords with our earlier observations, which showed that a reduction in the size of ND and increase in the size of CD in chewers with smoking habit and smoking habit alone than those with the habit of using chewers alone [13].
The results observed in the present study were, a clear proportional increase in CD was shown in those aged 25-45, a decrease in ND chewers users and a steady increase in N/C ratio from control individuals to chewers users. In addition to this age dependent increase in abnormal nucleus and cytoplasm ratio was observed in chewers.
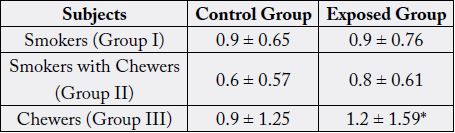
The frequency of micronucleus assay (MN) was studied in 30 building construction workers and in 30 controls. Workers revealed a significant induction of MN when compared with controls [p<0.05] [14]. individuals of the exposed as well as control groups with smoking habit, smoking with chewers and chewers alone showed an enhanced frequency of micronuclei in workers exposed and who chewers showed a highly significant increase [p<0.05] in MN frequency (1.2±1.59) were in control (0.9±1.25) [15]. Also in the smoking with chewers showed ahighly significant increase [p<0.05] in the users (0.8±0.61) were in controls (0.6±0.57).Workers also showed an increased MN frequency with an increase in duration of exposure [p<0.05]. A significant correlation was observed between MN induction and duration of exposure in building construction workers.
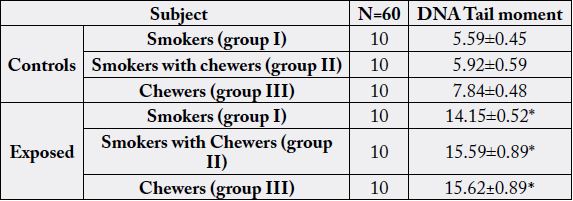
DNA damage was studied in a total of 60 groups using the Comet assay. The DNA tail moment will be compared by the exposed and control groups. In workers a significant increase (P < 0.05) in DNA mean tail length indicating DNA Damage was observed (p<0.05*). The results of DNA damage are given in Table 2. When compared with controls. In exposed group, a significant difference was observed between smokers and chewers and between smoking with chewers and chewers alone in relation to DNA migration (P < 0.05). DNA damage was further found to be chewers of exposed group workers are higher than the smokers with chewers and smokers alone from the exposedgroup’s (11.35±0.42) compare to the chewers of control group workers (10.48±0.58).Workers also showed an increased DNA tail movement with an increase in duration of exposure.
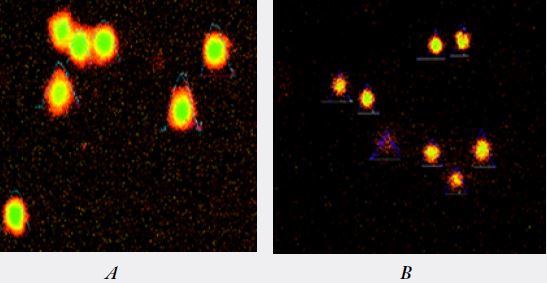
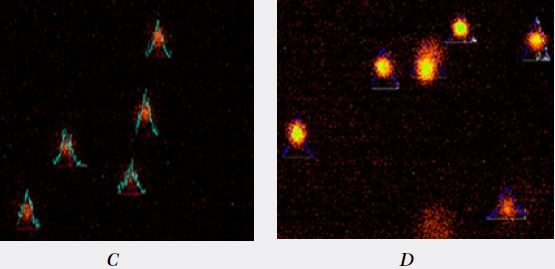
A (group 1) : Normal comet tail moment control.
B (group 2) : Comet tail moment observed in smokers.
C (group 3) : Comet tail moment observed in smokers with chewing habit.
D (group 4) : Comet tail moment observed in chewing habit.
The urinary levels of 1-OHP concentration of building workers of exposed group (11.35±0.42μmol mol-1 of creatinine) were significantly higher than the control (10.48± 0.58) displayed by the controls. A higher level of mean urinary level of 1-OHP was observed in the workers of exposed group in chewers (group III) in contrast to control group in chewers (group III). Similarly smoking with chewers (group II) also revealed an increased in mean urinary 1-OHP levels in workers when compared to their respective controls. A clear and statistically significant (P<0.05) increase in urinary 1-OHP levels were observed in experimental group when compared to control groups. As Analyzed chewers and smoking with chewers differences showed that exposed groups carry more urinary exposure than control groups.
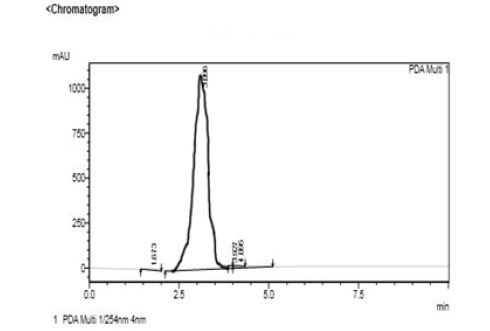
Fig.3 Representative Chromatogram for 1-Hydroxypyrene Standard with the following chromatographic conditions: Mobile Phase [water: acetone nitriyl (5:5v/v)]; Stationary phase [X-Bridge C18 (150 × 4.6mm) 5μm column]; Wavelength [254nm]; Flow rate [1mL/min]; Column temperature [30°C]; Injection volume [20μL].This graph represents the creatinine level of the chewers of the exposed group is the highly exposed and the value is 3.096 and the time interval is between 2.5 to 5.0 minutes. Then further study was GC-MS analysis for identifing the compound.
PAHs have been identified as cancer-inducing chemicals for animals and humans [16]. Also there is sufficient evidence that exposures in the occupational settings are carcinogenic to human. High occupational exposure to toxic substances such as PAHs and other petroleum products are the main toxicants to the exposed subject [17]. It is generally accepted that PAHs may cause direct/indirect genotoxic effects, thus genotoxicity biomarkers have a received a considerable interest as tools for detecting human genotoxic exposure and effects. Searching of association between biomarkers will help to select most advantageous biomarkers for further competent monitoring of various human exposures [2,18].
Occupational exposures to hazardous chemicals are common in industries using solvent based among the most common contaminants Polycyclic Aromatic Hydrocarbons (PAHs) have been identified as cancer inducing chemicals for animals and humans [19].
Occupational exposure to PAHs has been reported for its association with several cancers [20-23]. High levels of PAHs exposure in foundry workers have been reported earlier [24-26] and special PAHs, such as anthracene (Ant), fluorene (Flu), naphthalene (Nap) and phenanthrene (PA), are reported in foundries. To foresee total internal exposure to PAHs we used urinary level of 1-OHP as internal biomarker of occupational exposure.
Buccal cells are the primary barrier for the inhalation and are capable of metabolizing proximate carcinogens to reactive products [27,28,10]. Approximately 90% of human cancers originate from epithelial cells [29]. The oral epithelial cells represent a target site for early genotoxic events induced by carcinogenic agents entering the body via inhalation and ingestion. Exfoliated buccal epithelial cells were used to evaluate the genotoxic effects and are an efficient tool for biomonitoring studies [30,31].
Cigarette smoking is one of the daily life related public health threads that may influence the rate of cytogenetic damage [32]. Since cigarette smoke contains about 50 potent carcinogens, including polyaromatic hydrogen carbons (PAHs) and other organic chemicals, Hence, the increase in cellular and nuclear diameter by cigarette smoking is biologically believable.
In the present study, increased occurrence of abnormal nucleus and cytoplasm ratio was noticed in the exfoliated cells of the chewer’s users. Increase in age and extent of chewers use showed a significantly higher frequency of every investigated cytological changes. This finding is positively associated with oral carcinogenesis and support earlier investigations that disclose statistically significant decline in mean cytoplasmic area of cells taken from normal buccal mucosa of tobacco chewers [33,19,20,2]. The buccal mucosa collected from 25-45 years of age group chewer’s users also showed a significant result when compared to that of controls.
Increase in nuclear size is an indicator of cellular damage in tobacco users. Decreased cellular turnover as a result of prolonged chewers use following ageing would result in more number of mature cells with large nuclei in the smear [34]. This also accords with our earlier observations, which showed that a reduction in the size of ND and increase in the size of CD in chewers with smoking habit and smoking habit alone than those with the habit of using chewers alone [11].
The results observed in the present study were, a clear proportional increase in CD was shown in those aged 25-45, a decrease in ND chewers users and a steady increase in N/C ratio from control individuals to chewers users. In addition to this age dependent increase in abnormal nucleus and cytoplasm ratio was observed in chewers.
The main objective of the study was to evaluate if the exposure to complex mixture of chemicals in construction, induced increase in the level of genetic damage. The study was carried out in parallel with exposed and control group both from the same area and with similar individual characteristics. To evaluate the genetic damage two of the most common biomonitoring methods (Comet assay) were chosen. Biomarkers also permit enhanced analysis of health risk in humans exposed to carcinogens and because determinations are performed directly in human organism, uncertainties inherent in epidemiologic studies are avoided. There is no study available on the biomonitoring of building workers [35].
The current investigation reports genotoxicity in building workers from South India. The Comet assay test has been increasingly accepted as a reliable biomarker of genotoxicity in occupationally exposed groups [36]. The present investigation recommended that building workers under their particular conditions of exposure (tobacco) reveal clear evidence of genotoxicity. Our study revealed a significant induction of Comet tail length in building workers when compared to controls with respect to their age, smokers, smoking with chewers and chewers.
Similarly, an increased incidence of chromosomal aberrations was observed in the building workers with smoking habit (Shehla et al., 2012). These results are in agreement with our previous study which reported an increased chromosomal aberration among building workers [7] The building workers with smoking habits and tobacco chewing habit also shows more DNA damage, which shows tobacco has synergistic effect on inducing DNA damage.
In the present study, an elevated concentration of urinary level of 1-OHP was observed in the urine samples of PAHs exposed building workers. Similarly, 1-hydroxypyrene in urine has been used as a biomarker in studying occupational exposure to PAHs in building workers [31], coke oven workers [37], and foundry workers [38]. The urinary level of 1-OHP assay proved to be able to detect differences in PAH exposure not only at high occupational exposures but also in cigarette smokers [39]. The present study validated that the level of 1-hydroxypyrene in smokers sub-group and found an increased level of 1-OHP for combined exposure to PAHs [40-48].
Acknowledgments
The authors are thankful to the authorities of Karpagam University, Coimbatore, Tamil Nadu, India for
granting permission to use their facility and for their encouragements. We are thankful to the foundry
authorities, workers and control volunteers who came forward to provide their samples, without whose
cooperation the study would have been incomplete.
Conflict of Interest
None declared.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!