Biography
Interests
Raj Sewduth
Department of Oncology, Vesalius Research Center, - KU Leuven, Leuven, Belgium
*Correspondence to: Dr. Raj Sewduth, Department of Oncology, Vesalius Research Center, - KU Leuven, Leuven, Belgium.
Copyright © 2018 Dr. Raj Sewduth. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Endocytosis has recently emerged as a regulator of various pathways. Several of them are implicated in tumorogenesis. Vesicular trafficking of receptors can promote or down regulate pathways, depending on the type of vesicles (clathrin or caveolin dependent) or the end result of the endocytic process (recycling or degradation). Different pathways have been shown to be modulated by endocytosis, such as Wnt/ Erk/ P53/ Ras signaling. These pathways are activated in several types of cancers. Some receptors such as EGFR have even been shown to be mutated on residues essential for its endocytosis in specific cancers.
Introduction
Vesicular trafficking was first shown to be important in response to viral infection, immune response as well
as processes such as autophagy. Recent evidences have shown that endocytosis can also be important for
internalization of receptors, thus leading to activation of signaling pathways that play a role in oncogenesis.
Some oncogenes also appear to be trapped in vesicles leading to their inactivation, suggesting that endocytosis
could act as a tumor suppressor.
Main Text
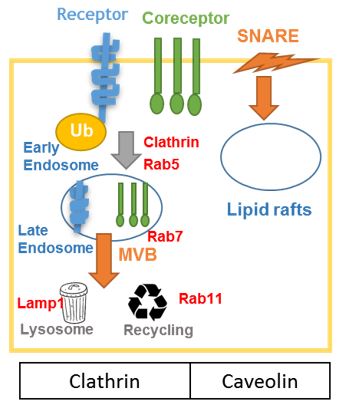
Endocytosis can mediate cell signaling in different ways by downregulate signaling by promoting degradation of receptors - as it is the case for some small G protein-coupled proteins that are internalized and fused with the lysosome for degradation. Endocytosis can also maintain signaling complex after their formation at the plasma membrane - as it is the case for activation of the mitogen activated protein kinase (MAPK) that leads to formation of the RAS-RAF-MAPK-ERK complex in endosome and activation of ERK. Endocytosis can also generate signal - as in the case of transforming growth factor - B receptor that is internalized into endosomes leading to recruitement and phosphorylation of SMAD2 leading to signal transduction [1-3].
In the case of Wnt signaling, endocytosis is required at different levels. Early endosomes would sequester GSK3-β in vesicles, leading to protection of its cytosolic substrates (β catenin or c-Jun) from phosphorylation, stabilizing newly translated β-catenin protein. Interestingly, GSK3-β would be recruited to the multivesicular endosome after binding to the Dvl/Axin-2 complex in the cytosol [4].
Endocytosis can also regulate the level of expression of Wnt receptors by targeting them to lysosome for degradation or recycling endosome for receptor recycling. Receptors are protected from degradation when they are sequestered in early endosome. The receptors can then only be restored to endosomal membrane via the recycling endosome. It is possible for membrane lipid components of the early endosome to recycle back to the plasma membrane but not to be degraded by fusion with the lysosome (back-fusion) [5].
Recent evidences strongly indicated that different ligands would induce different pathways via the same components by promoting endocytosis of the receptor complex via caveolin or clathrin mediated endocytosis. In the case of Wnt signaling, caveolin - dependant endocytosis of Fzd and Fzd correceptors would favor the Wnt canonical pathway, while clathrin - dependant endocytosis would favor the Wnt non canonical/ PCP pathway. First evidences were that Wnt3a induces the caveolin-dependent internalization of Fzd coreceptor, low-density-lipoprotein receptor-related protein 6 (LRP6) leading to recruitment of Axin to the cell surface membrane and to subsequent accumulation of B-catenin [6].
In contrast, Dkk1 induces the internalization of LRP6 with clathrin and inhibits Wnt3a response, reducing distribution of LRP6 in caveolin rich-lipid rafts [6]. In a similar manner, Dvl2 interacts with m2-adaptin, a subunit of the clathrin adaptor AP-2; leading to handling of Frizzled4 by the endocytic machinery and subsequent activation of the PCP [7]. These results echoes findings of Chen et al. 2003 who showed that Wnt5a induces endocytosis of Fzd4 to clathrin dependant vesicles, leading to recruitment of Dvl2 [8]. Recent evidence also show that upon binding of Wnt5a to Fzd7, Respondin 3 (Rspo3) binds to syndecan 4, leading to clathrin-mediated endocytosis of Fzd7 and activation of PCP in xenopus [9]. This body of evidence indicates that endocytosis of the Fzd complex via clathrin vesicles would favor the non-canonical pathway, while endocytosis in lipid rafts via caveolin would favor the canonical pathway.
Finally, the internalization of receptor complexes appear to be important during development and cancer. Drobolowki et al. suggested that the regulation of Wnt signaling through sequestration would be important in the formation of the dorsal axis during Xenopus laevis development. The vegetal pole of the egg would contain ‘maternal determinants’ such as Wnt-containing early endosomes that would be incorporated into multivesicular bodies (MVB) on the dorsal side of the egg. These MVB would sequester GSK3, making Wnt induced embryonic differentiation possible, leading notably to formation of the Nieuwkoop signaling center. Another example is the one of Rac that is internalized through Rab5, a process important for directed cell motility in drosophila, during motogenesis [10].
Several studies have also linked endocytosis and cancer. In some cases, endocytosis would attenuate activation of an oncogene, by promoting degradation. Aberrant activity of Rho small G-proteins, such as Rac1 and their regulators, is a hallmark of cancer and is modulated by endocytosis. Rac is internalized via lipid rafts/ caveolae when caveolin‐1 is phoshorylated, leading to its degradation and inhibition of subsequent signaling. Caveolin‐1‐deficient cells display increased Rac activation resulting in a more oncogenic profile [11].
In other case, endocytosis would activate oncogenes through the signaling endosome and recycling. The activity of the tumor suppressor protein p53 appears to be regulated by the ligand of Notch, Numb. Numb is located in the endosome and forms a complex with p53 and the ubiqutin ligase Hdm2. Numb inhibits the ligase activity of Hdm2, leading to the stabilization of p53 in the endosome. In line with this finding, low expression of Numb is a poor prognostic factor in breast cancer [12].
Mutations of signaling receptors are also modifications that occur in several cancer. In some cases, this can affect their endocytosis. Two mutations in EGFR appear to have a clear effect on its internalization. These genetics alterations were observed in glioblastoma, lung, prostate and ovary cancer [13-16].
Conclusion
These different findings identify endocytosis as a key player in oncogenesis, thus showing that targeting
proteins involved in trafficking is a valid strategy to impair tumor progression.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!