Biography
Interests
Ajita Naik MBBS1*, Mohamed Rahouma MD2*, Ihab Eldesoki MD3,7, Katherine D Gray MD4, Mohamed Kamel MD2, Maha Yehia MD3, Galal Ghaly MD2, Kritika Mehta MBBS5, Massimo Baudo MD6, Nagla Abdel Karim MD7 & Abdelrahman Mohamed MD2
1K. J. Somaiya medical College/Maharashtra University of Health Sciences, Mumbai, India
2Surgical Oncology Department, National Cancer Institute, Cairo University, Egypt
3Medical Oncology Department, National Cancer Institute, Cairo University, Egypt
4Department of Surgery, New York Presbyterian Hospital - Weill Cornell Medicine, New York, USA
5D Y Patil University School of Medicine, Navi Mumbai, India
6IRCCS Vita-Salute San Raffaele Hospital Via Olgettina, 60, 20132 Milan, Italy
7Medical Oncology Department, University of Cincinnati Cancer Institute, Cincinnati, Ohio, USA
*Correspondence to: Dr. Mohamed Rahouma, mhmdrahouma@gmail.com, Surgical Oncology Department, National Cancer Institute, Cairo University, Egypt.
*Ajita Naik MBBS & Mohamed Rahouma MD are equally contributed
Copyright © 2018 Dr. Mohamed Rahouma, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Lung cancer is a common cause of cancer death. Progression free and overall survival is challenging to predict due to the variety of underlying genetic defects leading to cancer development. A number of gene mutations and subsequent downstream pathway alterations have been identified in oncogenesis of non-small cell lung cancer (NSCLC), and specific genes like EGFR, KRAS, ALK, TP53 and genetic signaling pathways like PI3K/ALT/mTOR have been found to affect the prognosis and allow for options for targeted therapy. Moreover, there is evidence of high incidence of changes in receptors leading to resistance of the target sites to the available drugs causing deleterious effects on prognosis. Herein, we reviewed the available methods of diagnosing these changes at a molecular level and the implications diagnosing these alterations on survival. Literature suggests that singling out causative factors can be of prognostic value as well as serve as specific targets for therapy. Currently, a number of targeted therapies are under development. However, knowledge of genetic information opens opportunities to provide patients with a more accurate prognosis, specific treatment options, and the potential for improved treatment modalities.
Introduction
Lung cancer contributes heavily to cancer-related deaths in both men and women in the United States [1].
One of the major limitations of traditional chemotherapy and radiation is the balance between effectiveness
of the treatment and its deleterious effects on normal cells and thus the patient [2]. With advancement
in lung cancer genetics, the World Health Organization (WHO) classification of lung cancer has been
updated to reflect genetic and molecular mechanisms causing cancer [3,4]. Lung cancer is divided primarily
into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC). While SCLC are associated
with RB1 and TP53 mutations, NSCLC is associated with mutations in the KRAS, EGFR and TP53 genes
[5].
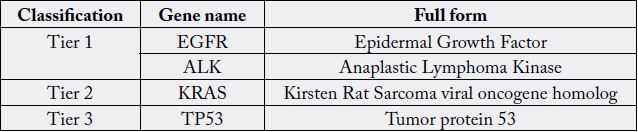
Mutations or copy number alterations in a given sample of tissue can be detected using targeted gene panels. These panels are designed to detect genetic abnormalities that are known to be most frequently associated with the cancer in question [6,7]. Next generation sequencing (NGS) is a cohort of technologies that can be used to sequence DNA templates [8]. The sample of tissue is analyzed using fluorescence in situ hybridization (FISH) and then tested against 50 key cancer-related genes. The mutations are classified into 4 tiers: tier 1 represents variants which have an approved course of action clinically, tier 2 denotes variants that do not have an approved course of action but can be used to help with clinical decision making, tier 3 includes variants that have been previously described in malignancy and tier 4 are variants that have not yet been described in malignancy [9]. A recent study conducted by Cheng et al. used NGS on almost 150 NSCLC tissue samples for mutations or singe nucleotide variants (SNV) in BRAF, EGFR, HRAS, IDH1, JAK2, KIT, KRAS, MET, NRAS and PIK3CA genes. They found that 36% of all patients who were staged IB-III had at least one of these mutations. [10] This identification of a significant number of actionable gene alterations provides options for specific treatment in patients with NSCLC [11].
Specific Genetic Mutations
The TP53 gene encodes for the p53 protein, a well-known tumor suppressor that is known as the “guardian
of the genome” due to its wide antiproliferative effects. p53 is usually latent and does not participate in
the progression of a normal cell cycle. Damage to DNA, oncogene activation or hypoxia activates p53
transcription which brings about downstream effects like apoptosis, cell-cycle arrest and autophagy [32-
34]. It is found to be the single most frequent gene involved with multiple cancers and many of the most
aggressive cancers including NSCLC have a mutated TP53 in as many as 80% of tumors, and p53 mutation
have been found to correlate with poor prognosis [35,36]. Traditionally, p53 mutants have not been drugtargetable.
However, there have been recent developments of compounds such as p53 reactivation and
induction of massive apoptosis-1 (PRIMA-1), APR-246 (methylated derivative of PRISMA-1), PK11007
(2-sulfonylpyramidine), PK7088 (pyrazoles) zinc mettalochaperone-1 (third generation thiosemicarbazone)
and peptides that are being developed with an expectation that they will be able to convert reactive properties
of mutant p53 into wild type properties. However, they are still under trial for their effectiveness in humans
[35].
Epidermal growth factor receptor (EGFR) is a transmembrane glycoprotein causing auto-phosphorylation
by intrinsic tyrosine kinase activity [12]. Studies show that NSCLC with EGFR mutations are associated
with higher mortality [13-15]. Epidermal growth factor binds to EGFR bringing about conformational
changes and phosphorylation of the intracellular domain. This leads to downstream activation of various
pathways including PI3K/Akt, Rafl-extracellular signal-regulated kinase, and activation of signal transducer
and activator of transcription (STAT) factors. Activation of these pathways leads to either cell proliferation
or apoptosis in normal cells; mutations in EGFR can thus be oncogenic and result in abnormal cell
proliferation or ability of the cell to avoid apoptosis [12,16]. Mutations in the gene coding for EGFR can
be detected by polymerase chain reaction (PCR). In NSCLC, alterations in the activity of genes coding for
EGFR have been reported in 43-89% of cases [17-19]. Besides mutations, amplification of gene expression
can also lead to similar effects in EGFR. FISH or chromogenic in situ hybridization (CISH) are used to
detect gene amplification [12].
Gefitinib and Erlotinib are two drugs that inhibit EGFR and have been recently approved for the treatment of NSCLC. They were found to inhibit the intracellular tyrosine kinase domain of the receptor [12]. Gefitinib and erlotinib provide a higher progression free survival period; however, there is no significant advantage in the overall survival when compared to chemotherapy [20]. In a recent study conducted by Zhang et al., they found that while the correlation between EGFR mutation and smoking was still controversial, the progression free survival with drugs that inhibit EGFR tyrosine kinase in patients with advanced NSCLC was better in non-smokers than in heavy smokers [22]. Peripheral blood or plasma can be used to test the EGFR mutations non-invasively, with a specificity of 88.5% and sensitivity of 64.5% by pooling data from thirteen articles [23].
EGFR-targeted therapy may also have implications for co-treatment. For instance, blockade of the immunosuppressive PD-L1/PD-1 pathway has had mixed results in NSCLC [24,25]. In one study, Lee et al. found the advantage of anti-PD-L1/anti-PD-1 inhibitor drugs like nivolumab and pembrolizumab limited to EGFR-wild rather than EGFR-mutant subgroup in their study [27].
KRAS is a member of the Ras family of proteins, which are small GTPases that participate in cell survival,
cell cycle progression, cell structure and polarity [needs citation]. These are regulated by guanine nucleotide
exchange factors (GEFs) and inhibitory GTP-ase activating proteins(GAPs) [28]. KRAS mutations are
most commonly seen in patients with a significant smoking history [29]. Mutations cause RAS proteins
to be insensitive to GAPs, which leads to persistent activation of downstream pathways and results in
abnormal cell growth, proliferation and survival [28]. Pao et al. observed that tumors with KRAS mutations
of exon 2 lacked response to kinase inhibitor drugs [29]. KRAS mutation is a poor prognostic indicator in
NSCLC due to aggressive behavior and escape from targeted therapy [30,31]. As opposed to previously
assumed theory that EGFR and KRAS mutations could be caused together, Gao et al. found that EGFR
and KRAS mutations are in fact mutually exclusive [31].
Rearrangement of the ALK gene can lead to NSCLC via expression of an oncogenic fusion protein. In
normal cell metabolism, the ALK gene is associated with regulating oncogenic receptor tyrosine kinases like
RAS-mitogen activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3K)/AKT and JAK-STAT
pathways. Mutations cause gene partners like EML4 and NPM fusion with the intracellular domain of the
receptor. As a result, abnormal proteins are expressed which promote atypical activity in the downstream
signaling pathways, leading to uncontrolled cell growth and survival [37]. The EML4-ALK mutation is a
subset of ALK mutations seen in NSCLC. These mutations can clinically express characteristic similar to
EGFR mutations. Shaw et al. found that 1 out of 3 patients who showed clinical characteristics similar to
EGFR mutation despite having a negative EGFR mutation in fact had EML4-ALK mutations [38]. ALK
mutations are estimated to be associated with 4-7% of all NSCLC and have poorer diagnosis than that
observed in NSCLC overall [39]. Drugs like crizotinib and ceritinib have been developed to act on the ALK
mutation target, although the development of tumor resistance to these therapies has limited widespread
use [39].
The PI3K/AKT/mTOR pathway is a prototypical oncogenic pathway in which signaling can be activated
by a number of factors including upstream mutations in EGFR and KRAS. Two to five percent of all of
NSCLC patients had a mutation in the PI3K/AKT/mTOR pathway. Interestingly, downstream activation
of this pathway plays a significant role in the acquired resistance and escape from treatment in therapies
targeting EGFR mutations.
Multiple compounds have been developed to target different components of this pathway. GDC 0941, BKM 120, BAY80-6946, and PX866 are potent PI3K inhibitors, whereas GDC0068 and GSK1241795 MEK1/2 inhibit different isoforms of AKT. Everolimus, temsirolimus and ridaforlimus bind to FKBP12 protein and attenuate the downstream effects of mTOR [40].
The SRC family of kinases (SFKs) consists of nine tyrosine-kinases and function to regulate progression
of carcinogenesis. They affect pathways which are associated with the development of metastatic tumor
potential. Abnormal expression of this family of genes has been implicated in various cancers, particularly
human epithelial cancers. c-SRC has been found to have a special correlation to the EGFR gene. Other than
NSCLC, SRC gene mutation has also been found to be contributory in breast cancers. EGFR association
causes c-SRC to become activated through various mechanisms such as Tyr845 phyosphorylation leading
to activation of multiple downstream factors including EGFR itself. There is a strong association between
SRC and EGF such that tumors having EGFR activity show increased SRC activity while inhibition
of c-SRC leads to apoptosis by decreased expression of downstream signaling. While Zhang et al. found
SFK activation to be associated primarily with NSCLC, Masaki et al found the incidence is higher in
adenocarcinomas. There was also evidence that depletion of c-SRC form cells reduces sensitivity to the
effects of compounds like gefitinib [41,42].
BRAF is a serine/threonine protein kinase that plays a relevant role in the mitogen activated protein
kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathway, involved in cell growth, proliferation,
survival and differentiation. MAPK/ERK pathway includes several proteins with kinase domains (RAF,
MEK, ERK) that convey signal transduction from membrane receptors to DNA in the nucleus of the
cell. The MAPK/ERK signaling pathway is evolutionarily conserved and critical in regulating cell growth,
proliferation, differentiation, migration, and survival [43]. An estimated 2.6% of NSCLCs have BRAF
mutations [44]. This effort has led to the development and Food and Drug Administration (FDA) approval
of several small molecule inhibitors targeting mitogen-activated protein kinase/extracellular-signal regulated
kinase (MAPK/ERK) pathway tyrosine kinase proteins such as BRAF and MEK [45].
Clinical Relevance of Gene Panel Testing
When tumor tissue is not available for sequencing, cell-free DNA can be for next generation sequencing.
However, in this context, the rate of mutated cells is less than 1% so NGS should report only clinically
specific and relevant genes. 50 gene panel have been developed while taking this into consideration [46].
Yamamoto et al. compared sensitivity and efficiency of NGS analyses with conventional analytical methods like PCR. They found the RNA-based NGS method to be clinically convenient and effective. They were able to use solid samples from biopsies as well as cytologic samples from bronchoscopic lavage and effusion draw.
NGS provided high sensitivity over a shorter duration (4 days) at a lower expense (US$144) per sample than conventional diagnostic methods. While the conventional method diagnosed 6 mutations including 6 types of EGFR mutation and 1 type of EML4-ALK mutation, NGS analyzed exon 15-23 of EGFR mutation, all CD regions of KRAS mutations and five kinds of ALK fusion mutations [47,48].
Mayo clinic recommends the following 50 genes in a gene panel to achieve the above aims:
ABL1, EGFR, GNAQ, KRAS, PTPN11, AKT1, ERBB2, GNAS, MET, RB1, ALK, ERBB4, HNF1A, MLH1, RET, APC, EZH2, HRAS, MPL, SMAD4, ATM, FBXW7, IDH1, IDH2, NOTCH1, SMARCB1, BRAF, FGFR1, FGFR2, FGFR3, NPM1, SMO, CDH1, JAK2, JAK3, NRAS, SRC, CDKN2A, PDGFRA, STK11, CSF1R, FLT3, KDR, PIK3CA, TP53, CTNNB1, GNA11, KIT, PTEN, VHL [49].
Conclusion
NSCLC heavily contributes to cancer related mortality. Next generation sequencing allows for detailed
knowledge of the underlying biology of each tumor, allowing for targeted therapy and the potential for
improved treatments. Newer techniques of determining the underlying abnormality like NGS confer higher
sensitivity in a shorter duration of time while maintaining cost-effectiveness. Additionally, this information
also provides better and more specific guidelines to determine prognosis of the disease.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!