Biography
Interests
Shimaa-Ahmed, M.1 , Hefnawy, Y. A.2 , Arafa, M. I.3 & Abd-El-Malek, A. M.2*
1Food Hygiene Department, Animal Health Research Institute, El-Minia Lab, Agriculture Research Center, Egypt
2Food Hygiene Department, Fac. of Vet.Med. Assiut Univ., Egypt
3Food Hygiene Department, Fac. of Vet.Med. Assiut Univ., Egypt
*Correspondence to: Dr. Abd-El-Malek, A. M., Food Hygiene Department, Fac. of Vet. Med. Assiut Univ., Egypt.
Copyright © 2022 Dr. Abd-El-Malek, A. M., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
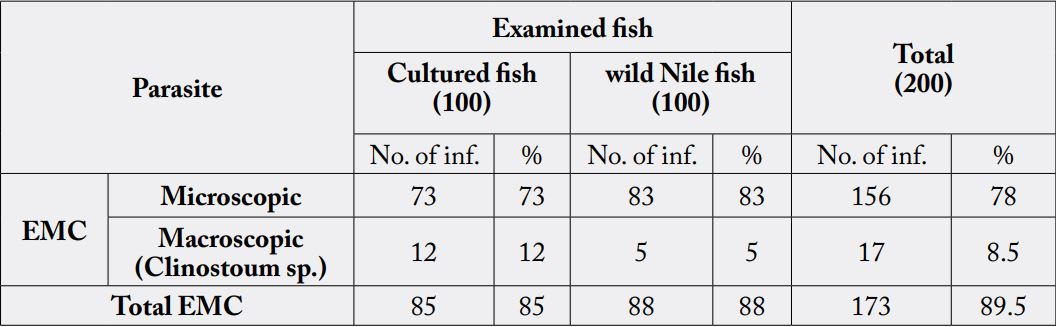
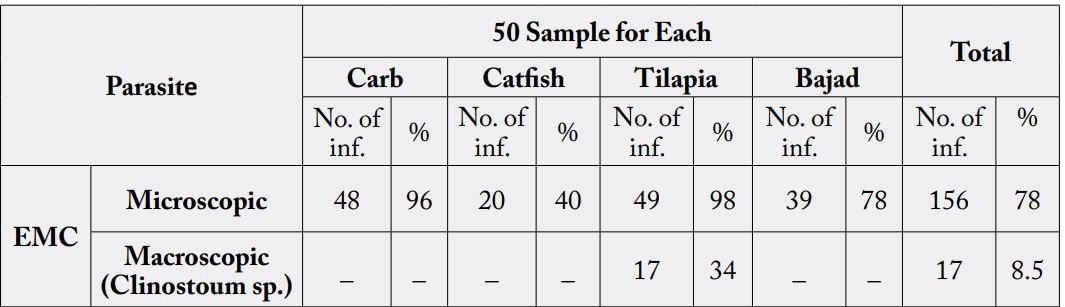
The present work aimed to investigate the prevalence of encysted metacercariae (EMC) in wild and cultured fresh water fish in El-Menia Governorate, Egypt. In addition, the effects of some processing methods on their viability were investigated. A total of 200 fish samples (100 wild and 100 cultured) included50 for each; Tilapianilotica (Oreochromisniloticus), catfish (Clariasgariepinus), bajad (Bagrusbajad) and carp fish (Cyprinuscarpio) were randomly collected from markets and some fish farms from El-menia city. Examination of encysted metacercariae (EMC) in the fish is commonly done by macroscopic examination and muscle compression technique.
The total prevalence of EMC among examined fish was 89.5%, it was 88% and 85% in wild fish and cultured fish, respectively. Microscopic EMC was detected in 83% and 73% of wild fish and cultured fish, respectively, while macroscopic EMC was detected in 5% and 12% of cultured fish and wild fish, respectively. Tilapia nilotica has the highest infection rate for both macroscopic and microscopic EMC in different examined fish species.
Microscopic E.M.C. was identified as: Cynodiplostomum E.M.C and Prohemistomum E.M.C while, macroscopic E.M.C identified as Clinostomum phalacrocoracis.
Effect of microwave cooking, acidic electrolyzed water (AEW) and mixture of sodium chloride with two concentrations of potassium lactate (2 and 4%) on infectivity EMC of Tilapia nilotica were studied through mice bioassay. The obtained results showed that infectivity of EMC was lost at high temperature of microwave cooking (400°c) for 2 and 5min. While other control measures observed significant reduction in infectivity of EMC but not completely lost The data were statistically analyzed. The current study revealed a high prevalence of zoonotic EMCs in different examined fish, which represents a potential risk to public health if consumed raw or improperly cooked. In conclusion, thorough microwave cooking was effective in reducing risk of encysted metacercariae in fish muscles.
Introduction
Worldwide, about 40 million people suffer from trematode infections associated with the consumption of
raw or undercooked fish products [1,2]. Many research described fish as a source of serious parasitic disease
which may be harmful for human or fish eating mammals if it consumed raw or improper cooked [3,4].
Muscles of infected fish with encysted metacercariae had also some alterations on their chemical composition. [5] Ghada et al. mentioned that heavy infection of fish muscles with EMC induced a significant decrease in protein, oil and ash contents with a significant. Also they noticed increase in the total volatile nitrogen, thiobarbituric acid number, peroxide value and free fatty acids in muscles of infected fish.
Heat inactivation of parasites proved effective for eliminating the risk of parasitosis but trematodes possessed a higher heat resistance [6]. The microwave has microbial destruction effect as it inactivate microorganism directly by heat [7]. Food ingredient absorb energy from the microwave (dielectric heating process) as circling molecules hit other molecules make motion which diffuse energy distributes as vibration which raise the food temperature [8].
The effects of different concentrations of salt, vinegar and acetic acid for different exposure times on viability of EMC were studied previously. [9] Ammar and Arafarecorded that dry salting of O. niloticus could kill the EMC within one hour of saltcontact, while marination in 5% acetic acid for one minute resulted in eradication of EMC after 2 hours of treatment.
In recent years electrolyzed water (EW) is used as an alternative of traditional heat treatmentsfor controlling pathogens. It has a wide range of food applications, including dipping and spraying [10]. It is safe for skin and mucous membranes, easy to handle and of low production cost [11].
Therefore the present study aimed to:
- Investigate the prevalence of the different encysted metacercariae in wild or culture fresh water fish at El-Menia Governorate.
- Study the effect of microwave, acidic electrolyzed water (AEW) and mixture of sodium chloride and two concentrations potassium lactate on the infectivity of EMC through mice bioassay.
Materials and Methods
From March 2021 to Feburary 2022, a total of 200 fish samples (100 wild and 100 cultured) were collected
randomly from different localities and from some fish farms at El-menia Governorate. The obtained
samples were included tilapia (Oreochromis niloticus), catfish (Clarias gariepinus), bajad (Bagrus bajad) and
carp (Cyprinus carpio) fish (50 of each). Fish samples kept in ice box and examined directly at El-menia
regional Lab Animal health research institute.
-Macroscopic Examination
The specimens under investigation were carefully examined by the necked eye for the detection of macroscopic
metacercaria in musculature and gills [12].
-Microscopic Examination for Detection of Encysted Metacercariae
From each fish, snips (about one gram each) were taken from different muscles of the fish, especially head,
near dorsal fins, tail regions. Muscle snips were compressed between two glass microscopic slides and
examined under binocular dissecting microscope to detect EMC and to determine viability by observing
their characteristic rotator movement [13]. The recovered encysted metacercariae in the fish flesh were
prepared for further experimental study.
AEW (pH of 3.4 and free available chlorine ACC of 30ppm) was produced by electrolysis of tap water
brined with sodium chloride (3%) through the electrolysis chamber [14]. The pH level of formed acidic electrolyzed water was estimated using a digital pH meter (FSSAI, 2015). Also, ACC was estimated by
chlorine test kit, according to [15].
-Pieces of heavily infected fish muscles (100 gm) were dipped in, PH (3.4) of EW for 15 minutes then thieved and cooled at refrigerator (4°C) for 8h.
Study the Effect of Microwave Cooking on the Viability of EMC
Pieces of heavily infected fish muscles (100gm) cooking in a microwave oven (700 W) at high temperature
(400°) for 2 and 5 minutes in accordance with the recipe provided by Fresh factories [16].
To detect the most applicable salts concentration, the experiment design into two treatment groups. The
first one is composed mixture of sodium chloride 2% and potassium lactate 2%. The second treatment is
composed mixture of sodium chloride 2% and potassium lactate 4%.
Pieces of heavily infected fish muscles (100gm) were soaked in corresponding treatments for 15 minutes and later cooled at 4°C for 8 hours before experimental work [17].
-After each treatment was done, the digestion for fish muscles samples was appliedas described by [18] Garcia to obtain the suitable number of EMC that used in mice bioassay
Experimental Infection of Laboratory Animals
A total of 28 albino mice weighing 200-300 gm reared one week and their feces were examined daily for any parasitic infestation to insure it is free from parasites then divided to 7 groups:
- The first group was infected with 250 EMC as a control positive group.
- The second group was infected with 250 EMC after treated with microwave at high temperature for 2m.
- The third group was infected with 250 EMC after treated with microwave at hightemperature for 5m.
- The fourth group was infected with 250 EMC treated with potassium lactate (2%).
- The fifth group was infected of which 250 EMC treated with potassium lactate (4%).
- The six one was infected with infected of 250 EMC treated with AEW (pH 3. 4).
- The last group mice were left uninfected as negative control groups.
Mice were infected experimentally with (250) encysted metacercaria, in accordance with local Institutional Animal Care and Ethics Committee (Assiut University, Egypt. Recovery of adult worms from the experimentally infected mice was done after egg detection in feacal examination. Positive infected mice were sacrificed within 10 days from the day of infection to collect and counted under a microscope, as in previous studies [20].
Statistical Analysis
Statistical analysis was performed using Graph Pad Prism 8.0 software (Graph Pad Software Inc., San
Diego, CA, USA). The data were analyzed using the chi-square test or Fisher’s exact test to assess the
association between prevalence and risk factors. While “P” value of <0.05 was considered statistically to be
significant [21].
Results
The total infected rate of encysted EMC in examined fish was 89.5% (173/200), where microscopic EMC
was detected in 78% (156/200) and macroscopic EMC were infected in 8.5% (17/200) of examined fish.
Infection rate in wild fish and cultured fish was 88% &85% respectively. Infection rate of cultured fish with
microscopic metacercariae was 73% (73/100) and in wild fish was 83% (83/100). Macroscopic metacercaria
was detected in 12% (12/100) of cultured samples, and 5 % (5/100) of wild fish samples (Table 1).
Morphologicaly microscopic EMC differentiated into:
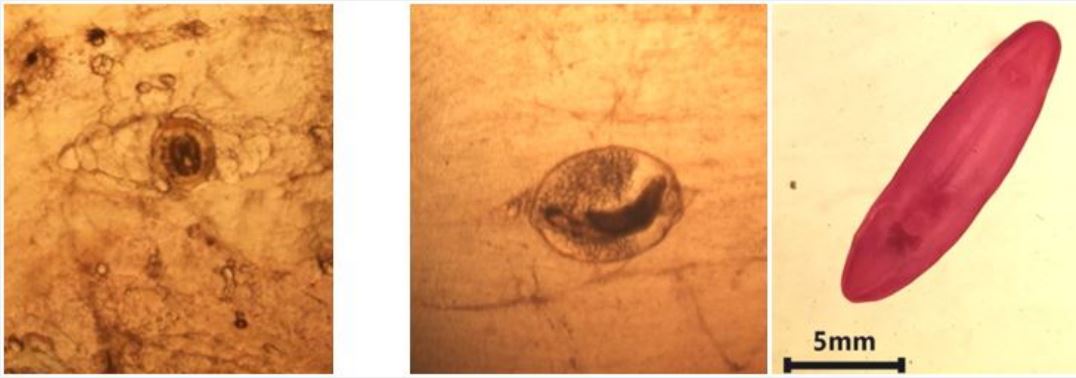
The macroscopical EMC were found in the branchial and pharyngeal regions of Tilapia spp. only and their infection rate was 34% (17/50). Morphologically macroscopic EMC differentiated into:
1-Microwave cooking of fish (700/W) at high temperature for 5 minutes completely destroys the encysted
metacercariae used in experimentally infection, where mice were free from any adult trematodes. Also,
microwave cooking at 700/W at high temperature for 2 minutes destruct most EMC inoculated in
experimentally infected mice, and where the infectivity rate was 0.40% (Table 3).
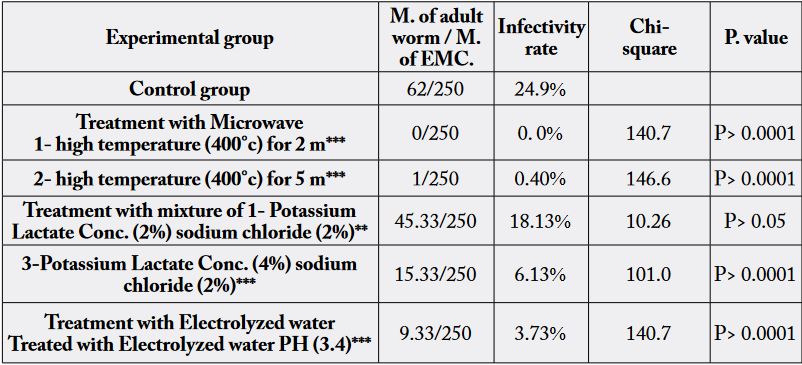
2- Regarding the effects of acidic electrolyzed water (EW) on the viability of the encysted metacercariae, mice inoculated on EMC treated with acidic EW (PH 3.4) revealed that EW was not sufficient to completely destroy EMC in examined fish sample. The infectivity rate was 3.73% in experimentally infected mice (Table 3).
3-Different concentrations of mixture of sodium chloride and potassium lactate were not sufficient to completely destroy EMC in examined samples. The affectivity on viability of EMC was increased by the concentration of potassium lactate. The infectivity rate was as follows: sample treated with mixture of sodium chloride (2%) and potassium lactate (2%) was 18.13%, while sample treated with mixture of potassium lactate (4%) and sodium chloride (2%) was 6.13% (Table 3).
Discussion
Parasitic infection of fish in tropical and subtropical countries represents a serious problem for aquaculture
as result of their huge economic losses [22]. They affect growth rate especially for young fish as well as they
lose their market value [23]. The presence of metacercariae is very common especially in freshwater fish and
they may cause public health problems [24].
In this study the overall infection rate of EMC in examined fish was 89.5%. This wide variety of EMC in different species of examined fish reflected the presence of intermediate and definite hosts in our study region. The present results was relatively similar that obtained by previous researchers as [25] in Assiut Governorate and [26] in Giza Governorate, who reported infection rate with different EMC was 84.75%, 84.8% and 82.8% respectively. On other hand, the present results considered higher than those reported by [27,28] they recorded that the prevalence of EMC in fish was 44.6%, 23.2%, and 56.3% respectively. Such variations in the frequency EMC may be attributed to various factors including the locality from which fish were caught and the degree of water pollution with human, animal and birds excreta and feeding habit of fish. [29] Skinner reported any environmental stress or chronic exposure to pollutants leads to release of corticosteroids which are immunosuppressant so fish become susceptible to any pathogenic organism.
In the current study, infection rate of microscopic encysted metacercaria was relatively higher in wild fish than cultured fish; it was 83% &73% respectively. This result agree with [9] who mentioned that the prevalence rate of microscopic EMC was 88.3 %and 26.7% in wild fish and cultured fish respectively in Assiut Governorate. Difference of percentage between wild and farmed fish may be attributed to fish raised in rivers, canals, streams and lakes may be more exposed to infect snails/cercariae, and reservoir hosts, compared to fish raised on farms in farms, where the pond environment may not favor the presence of snails [30].
Contrary to this result was recorded by [31] Eissa et al. who reported that the infection rate in farmed and wild fish in Sharkia Governorate was 67.33% and 42.33% respectively. Also This may attributed to overcrowding in some fish farms, which affects the immune status of the fish as a result of increase environmental contaminants in these farms [32].
In the current study, prevalence of microscopic EMC was varied according fish species. The highest infection rate of microscopic EMC was detected in Tilapia“O. niloticus”(98%) and the lowest infection rate with EMC was detected in cat fish “C. garpienus” (40%).This result cleared that tilapia “O. niloticus” thought to be the main second intermediate host for trematodes EMC in Egypt. This result is nearly similar to those given by [25].
Difference of infection rate between fish species may be attributed to the difference in the habitat, food supply and abundance of both aquatic snails and the aquatic birds which play the main role to complete the life cycle of some trematodes and climatic variations of the fish sampling areas [25].
Microscopic metacercarae recovered in the present work were identified as Prohemistomatide and Cyn. odiplostomatide, their morphologically coincided with that previously reported by [12,26,33].
In other hand, macroscopic encysted metacercaria was identified as Clinostomum phalacrocorais. It was detected only in Tilapia niloticus and their total infection rate was 34%, it Cl. Phalacrocoracis metacercaria was recorded as a parasite only for Nile tilapia and known as Yellow grub [34]. It was found previously in Egypt by; [9] Ammar and Arafa they detected it in 19.2% in examined wild O. niloticus and they not detected it in farmed Tilapia. [35] Manal et al. described it as yellow cyst in 39.16% of O. niloticus from the River Nile at El-Minia District. Also, [36] Mai et al. recorded that the infection rate of Cl. Phalacrocoracis EMC among the investigated O. niloticus in Egyptian water was 60.93%. Regarding to the morphological characters, description of Cl. phalacrocoracis detected in the present work agree with [36,37].
As shown in table (3) microwave cooking by 700/W at high temperature for 5 & 2 minutes were sufficient
to destroy all EMC in muscle of infested fish, where intestine of mice fed on microwave cooked fish were
free from any adult trematodes. Our result agrees with many authors; [38] determined that microwave was
very effective on larval stage of Opisthorchis metacercariae and it destroyed it completely. [39] Panupan et
al. found that a heating process, by microwaving at 400 or 800 W for at least 5 min, could kill metacercariae
in all sizes of fish. Also [40] revealed that using microwave 500 W/2 minutes was enough to destroy EMC.
Heating process of microwave ovens depending on water content, the depth of initial heat deposition may
be several centimeters or more and it cook food “from the inside out” [41]. The microbial destruction by
microwave done by; 1- inactivates and destruct microorganism directly by heat [7]. 2- dielectric heating which
means water, fat, and other elements in the food absorb energy from the microwaves, circling molecules hit
other molecules and put them into motion, thus diffusing energy, this energy, distributed as molecular spins,
vibrations and/or translations raises the temperature of the food [8]. [42] Ali and Al-Mahmoud recorded
that the effectiveness of heating depends on several factors, such as, species of parasite, fat content and fillet
thickness of fish.
Dry salting was the most effective processing mean to kill the EMC in O. niloticus muscles within one hour [9]. The current study investigated effect of two concentrations of potassium lactate in mixture with sodium chloride for 15 minutes on infectivity of EMC. Data summarized in Table 3 verify that both of concentrations used had significant values on the viability of EMC compared to control, but not sufficient to destroy all EMC in examined fish muscle. Previous studies of anti-parasitic effect of mixture (sodium chloride and potassium lactate) indicated that mixture of (2% sodium chloride and 1.4% potassium lactate) was able to prevent transmission of T. gondii experimentally either in cats or mice [17,43].
Electrolyzed water (EW)is mainly divided into two different types, acidic(AEW) and alkaline(BEW), AEW has a very powerful effect as a disinfectant. There are several factors affects antimicrobial activity of EW; the available chlorine concentration (ACC), the pH value and the oxidation reduction potential (ORP) in addition to storage time [44].
Regarding to the effect of AEW on the infectivity of EMC; in the present work, infective rate of EMC was significant decreased (3.73%) in mice group fed on fish muscle treated with acidic Electrolyzed water pH (3.4) as shown in Table 3. According to the available literatures, the present work is considered the first study of anti-helmenthic effect of acidic electrolyzed water(AEW). While anti-protozoan effect of electrolyzed oxidizing water (EOW) was recorded by [45] who mentioned that administration electrolyzed oxidizing water (EOW)in mice experimentally infected with Trypanosoma cruzi showed a beneficial effect on parasitaemia, general physical condition, and mortality. On the other hand the effectiveness of acidic electrolyzed water (AEW) in reducing airborne microorganisms was investigated previously by [58] they mentioned that slightly acidic electrolyzed water spray can used as potential method for reducing microbial presence in layer houses.
Conclusion
Our results cleared high incidence of different encysted metacercaraie in muscles of examined fresh water
fishes in El-Minia Governorate. This needs to raise awareness in fish health management and application
of suitable control measures. According to mice bioassay study; microwave cooking was the best way to kill
metacercariae in fish muscle. Furthermore, AEW and mixture of sodium chloride and potassium lactate
(different concentrations) gave significant values for inactivation the EMC in fish muscle.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!