Biography
Interests
Vaishnavi Pandey1, Ajai Singh1*, Sabir Ali2, Amit Kumar Gond3, Salma Siddiqui4, Manish Yadav2, Archana Raikwar1 & Anamika Singh1
1Department of Paediatric Orthopaedics, King George’s Medical University, Lucknow, India
2Department of Orthopaedic Surgery, King George’s Medical University, Lucknow, India
3Department of Paediatrics, King George’s Medical University, Lucknow, India
4Department of Biochemistry, King George’s Medical University, Lucknow, India
*Correspondence to: Dr. Ajai Singh, Department of Paediatric Orthopaedics, King George’s Medical University, Lucknow, India.
Copyright © 2021 Dr. Ajai Singh, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Congenital Talipes Equinovarus (CTEV) is the most frequently reported congenital abnormalities that influence children’s lower limbs in particular. With the prevalence of 1-2 percent per 1000 births worldwide, it occurs mainly in males as opposed to females in a 2:1 ration and is bilateral in about half of cases. The etiology of CTEV can eventually play a role in determining the prognostic and the choice of therapeutic interventions for an individual patient. According to available literature, there was the number of risk factors reported to be associated with its occurrence. However, some of them are popularly known to the world, while some still remained latent. CTEV has been positively linked in the latest literature with the genes of Homeobox family, collagen-family, GLI3, T-Box family, muscle contractile family and apoptotic pathway genes mainly due to its unknown etiology The recent advances in clubfoot genetics are aimed at examining and explaining the impact of how a demographic, environmental and genetic classification involves itself and how it could result in advanced strategies for individualized therapy. Also, CTEV is thought to be associated during pregnancy with certain maternal environmental interferences, dietary intake, metabolism including mainly folate metabolism, and related activities of the promoter-inducer gene. Hence, the principle objective of this study is to get an overview of all the known and latent risk factors of CTEV, based on the available studies and then update the information as well as hypothesis accordingly.
Abbreviations
CTEV: Congenital Talipes Equinovarus
MTHFR: Methylene Tetra Hydro Folate Reductase
HOX: Homeobox Gene
PITX1: Paired Like Homeodomain 1 Gene
CASP10: Caspase 10 Gene
COL9A1: Collagen Type 9 Alpha 1 Chain
NAT2: N-Acetyltransferase 2
DNA: Deoxyribonucleic Acid
HPA: Hypothalamic Pituitary Adrenal Axis
UK: United Kingdom
ANC: Antenatal Care
CVS: Chorionic Villus Sampling
Introduction
Clubfoot is one of the most prevalent congenital limb deformities, also called congenital talipes equinovarus.
It is often an isolated congenital condition and is believed to be idiopathic [1]. It can also be acquired and
is referred to as A CTEV i.e. Acquired Equinovarus Congenital Talipes (Table. 1) [1-3]. It appears with
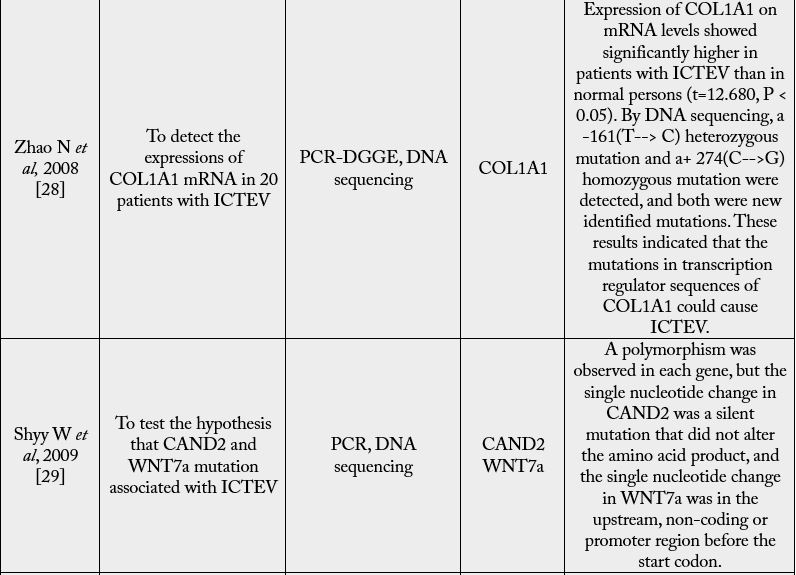
a proportion of 2:1 between males and females and is bilateral in about half of the cases [4,5]. Congenital
talipes equinovarus (CTEV) affects 1-2 per 1,000 births worldwide. This 3-D deformation is recognizable
after delivery. The foot is held in a fixed equinus with adduct us and cavus of the midfoot and a varus of the
hindfoot [4].
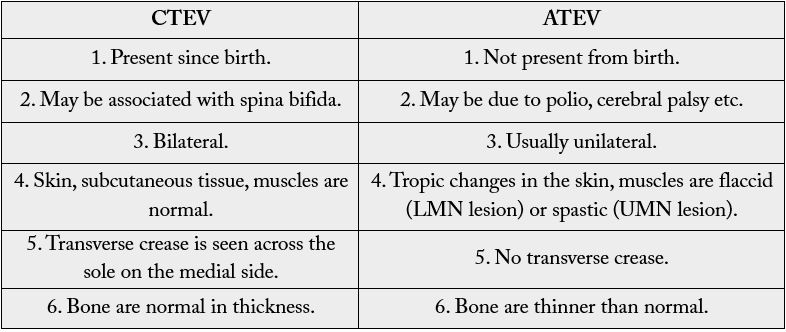
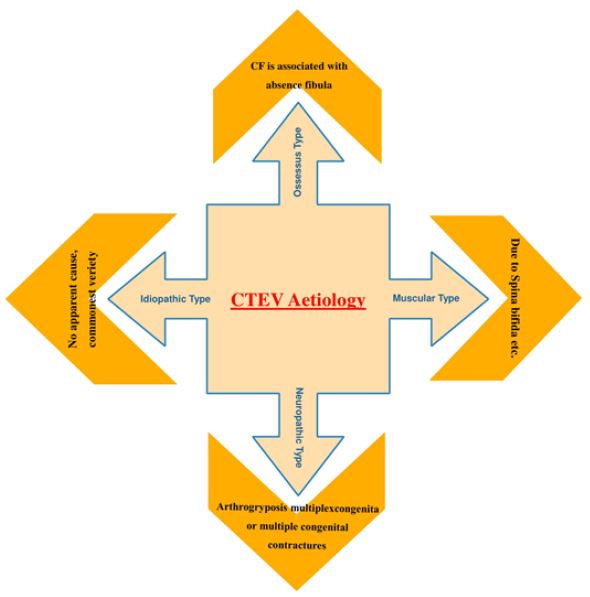
In some cases, CTEV may occur as part of a genetic syndrome in combination with other characteristics or congenital anomalies, but in the majority of cases, it may occur in isolation and is considered to be isolated CTEV (Figure 1) [3,4]. CTEV’s etiology is not yet known [5]. There was no clarification of either the environmental or genetic factors [6]. Mutations in genes involved in limb and muscle development are risk factors for clubfoot [5], specifically those encoding the contractile muscle complex and those regulating the expression of these many genes [6]. Common genetic variants such as HOX homeobox genes, insulin such as protein binding growth factor, MTHFR gene and Caspase gene family have either been reported to be directly associated with isolated clubfoot or to regulate the expression of other genes involved. According to the latest research by Wang et al. HOXD-12 and 13 gene is liable to cause congenital talipes equinovarus [7]. Many studies showed that smoking cigarettes are one of the most significant and coherent determinants in raising a child’s risk for CTEV during pregnancy [8]. In the presence of a favorable history of CTEV, smoking also raises the danger to 20 times, thus supporting the function of the gene in CTEV [9]. Environmental factors have been suggested as contributing to CTEV development, such as a small uterine cavity, mother-ingested drugs during pregnancy and herbicide aerial spraying [10].
A clubfoot prenatal diagnosis gives parents the opportunity to know about treatment and prognosis in advance, including prenatal counselling services, and this may allow clinicians to set up their network for optimal management of the disease. [11,12] However, the accuracy of this examination depends on a number of factors, including physician experience, gestational age, equipment quality and methodology [12].
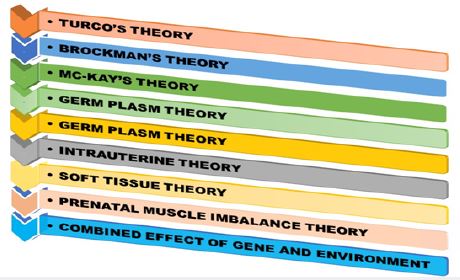
Etiology of Clubfoot With Its Management Trends
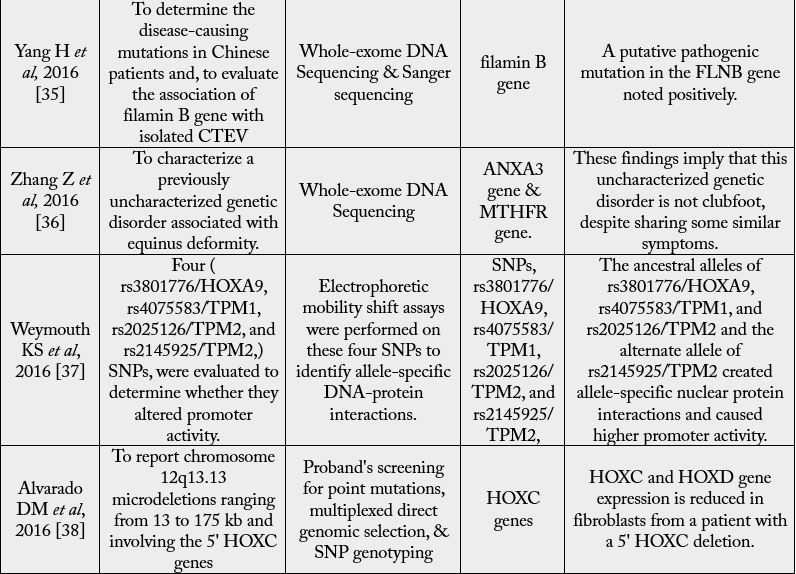
CTEV is by far the most prevalent congenital foot defect that gives club-like appearance [1] In clubfoot
patients the prevalence of congenital anomalies or chromosome abnormalities varies greatly from 24% of 50% according to population [13]. According to the Turco’s theory medial displacement of navicular and
calcaneus around the talus could be the cause of CTEV, according to Brockman’s theory the congenital
atresia of the talonavicular joint could be the cause of CTEV, according to the Mc-Kay’s theory the threedimensional
bony deformity of the subtalar complex could be one of the cause behind CTEV [12,13],
and etc. Furthermore, there are also many more theories emphasizing the link between mother-child
and deformity, including The Intrauterine theory which states that CTEV could be due to compression
by malposition of the fetus in utero and The Prenatal muscle imbalance theory which states that CTEV
could be due to weak pronators and overacting extensors and inverters etc (Figure 2). With advancing
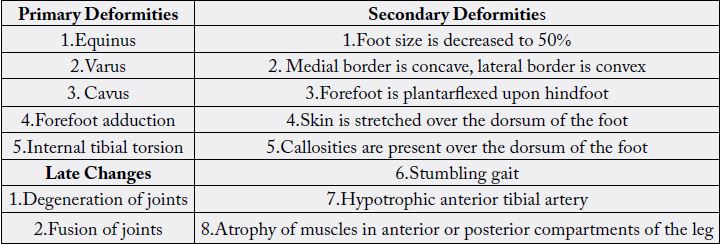
age, the cosmetically unsightly clubfoot starts posing functional problems like altered gait (stumbling gait),
callosities, and degeneration and arthritic changes in the ankle with foot joints. CTEV can be assessed by
Dorsiflexion Test, Plumb Line Test and Scratch test etc. Pirani’s classification is the most accepted findings
for severe abnormality [14]. Since CTEV is a mechanical problem (Table. 2), radiography is by far the most
important investigation. However, as per the recent studies, the laboratory data actually help us to find the
positive link between genetic malfunctioning and occurrence of CTEV. This, in turn, forces us to establish
a new approach of laboratory diagnostic parameters too [11,14]. CTEV can be managed by conservative
management, surgical management. It is the treatment of choice in infants less than 6 months of age.
Ponseti in the year 1950 described a very effective conservative method of treating clubfeet with very few
recurrence rates. The Ponseti method is now successfully used to treat severe non-idiopathic deformities of
the clubfoot [8,12].
Environmental Interference in Occurrence of Clubfoot
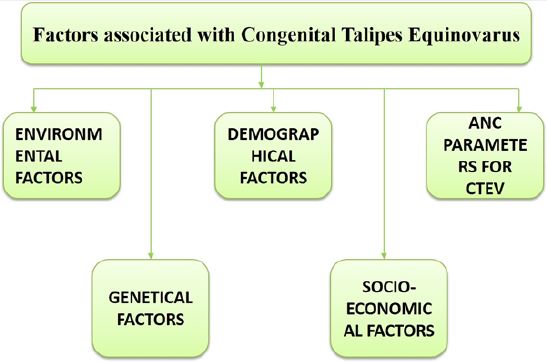
Multi-factor involvement, including genetic and environmental factors, has been proposed by epidemiological
studies to contribute collectively to CTEV etiology (Figure 3(a) & 3(b)) [14]. Various studies have shown a
greater risk of CTEV in infants due to smoking during pregnancy because it involves an enhanced possibility
of other birth defects like limb reduction defects, abdominal wall defects, and certain heart defects [15]. In
the absence of a club foot’s family history, maternal smoking exposure is associated with isolated clubfoot.
Vascular disruptions or compromise associated with smoking can be a probable factor contributing to the
incidence of clubfoot.
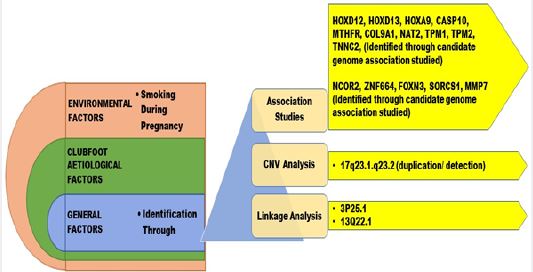
The potential for gene-environment interaction in clubfoot etiology on the Asian population has not been
examined yet. Indeed, available researches also demonstrate that both maternal smoking and the history
of the family are essential causes for clubfoot and that these two variables can have a substantial potential
relationship. It also offers a further indication of etiology of clubfoot and emphasizes the significance of
considering interactions of sex, laterality, and higher-order in etiological studies of defects.
Genetic Mis-Functioning as a Reason Behind CTEV
Genetic factors make a substantial contribution to CTEV etiology. The genetical cause indicated that
CTEV is prone to segregate in families [16]. As per the available literature, it has been found that there is a
link between family history of CTEV, with the occurrence of CTEV in neonates [17,18]. The significance
of genes involved in the growth of premature limbs was recently identified by the discovery of an unusual
mutation in the transcription factor PITX1 [19]. PITX1 is the first gene involved in clubfoot explaining the
foot’s unique involvement [19]. Mainly Hox-Gene Family, MTHFR Gene, Caspase-Gene Family, etc. are
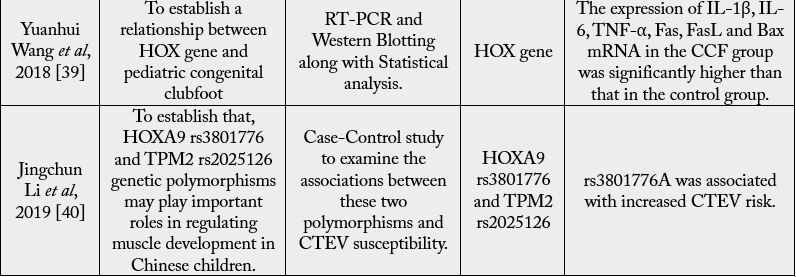
the genetically identified factors through associative studies (Table. 3). Genes with apoptosis, homeobox A
and D (HOXA and HOXD) and the genes involved in muscle contraction, have suggested the contribution of genetic in the accountability of CTEV [20,21]. Heck and colleagues [22] detected evidence that
a rare CASP10 gene allele was linked and associated with simplex CTEV cases. Polymorphism of
methylenetetrahydrofolate reductase gene (MTHFR) in mothers was also associated with CTEV [21]. The
gene COL9A1 is the significantly susceptible gene for CTEV, as the elevated expression of COL9A1 was
noted relative to ordinary people [20]. NAT2 (N-acetyltransferase 2) contributes to the biotransformation
of N-acetylation tobacco smoke [23]. Functional analysis has shown that the variants associated with CTEV
result in allele-specific interactions between nuclear proteins and cause higher promoter activity [9].
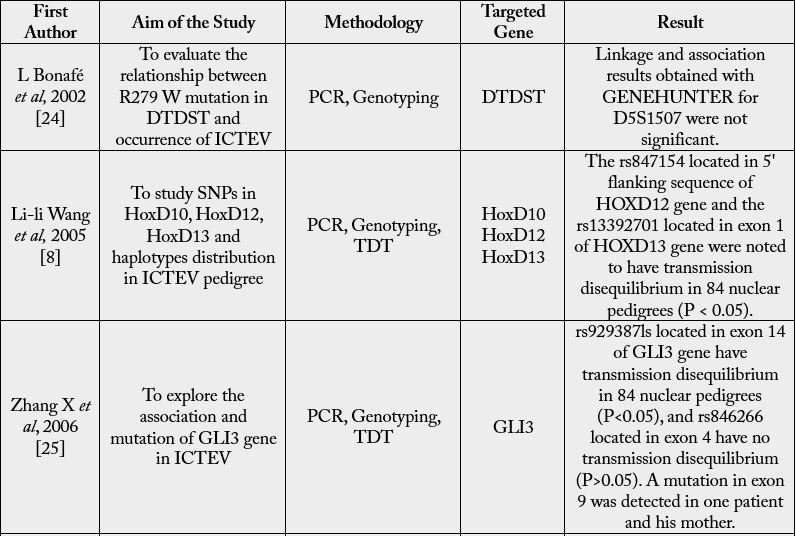

For many areas of the body, the HOX gene plays a vital function, including the nerves, muscles, bones,
and blood vessels. Thus, diseases can occur on the limbs if any HOX gene has mutations or an irregular
expression HOXD12 and HOXD13, which are the primary susceptible genes of CTEV also. Genes in
the HOXA cluster, i.e., chromosome 7p15, chromosome 2q31,33, and chromosome 12q13.13 are engaged
in the patterning of the limb, muscle and axial skeleton [41,42]. A deletion in its region is thought to be
associated with clubfoot. The HOX family comprises 39 genes in four A, B, C and D clusters. Ester et al. and
others [30,43] have shown that variations of the HOXA, HOXC, and HOX-D genes in regulatory regions
are correlated with CTEV.
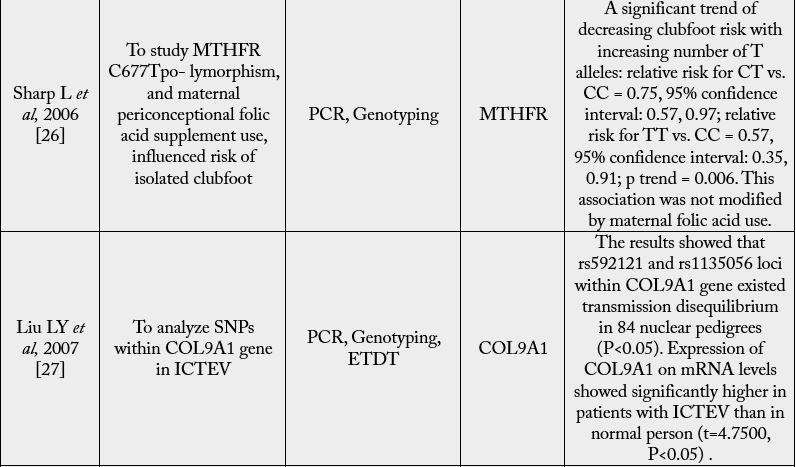
MTHFR refers to methylenetetrahydrofolate reductase. It causes genetic mutation, which can lead to high
blood homocysteine levels and low folate and other vitamins. A maternal genotype MTHFR did not affect
the clubfoot risk for the children as a whole, although a possible association with the use of folic acid
has been reported as associated with the CTEV risk. This is the first recognized study on unique genetic
polymorphism in clubfoot [33]. Many essential metabolic processes include B vitamin folate, including the
synthesis and repair of DNA and DNA methylation [43]. The risk of multiple congenital malformations
has been observed in pregnant women with poor folate status [30,33]. MTHFR played a part in etiology
of many congenital malformations - which includes neural tube defect and orofacial clefts - but, there is a
paucity of literature on clubfoot in the Asian population [33]. According to the latest research, researchers
proposed that there was an association between the use of maternal genotype, consumption of folic acid and
risk of clubfoot in the embryo. However, interaction tests have not yet been clinically established.
Demographic Factors Accountable for Clubfoot
There are a number of population-based orthopedic-confirmed clubfeet patients and controls for assessing
the incidence patterns [26,27,44]. The male majority reported in various studies was reported in our study, including the demonstrations and analysis for isolated cases, those that were associated with other chief
malformations, unilateral and bilateral cases, and that could be attributed to fetal constraint or genetics.
However, the lowest magnitudes of masculine births have been observed among cases of further major
congenital malformations, those associated with fetal constraint, and those with a positive family history in
first-degree relatives, possibly due to etiological heterogeneity among them [44].
There is evidence in both animals and humans that psycho-social and environmental stressors modify the sex
ratio in favour of females, possibly by activating the hypothalamic-pituitary-adrenal (HPA) axis [44]. Why
more males are born with clubfoot than females remains a mystery, with countably low available literature
and hence requires further studies with special emphasis. However, we may suggest that the environmental
or psycho-social factors that change the male sex ratio may be worth considering as a risk factor [24].
Maternal age was reported to be inversely linked to clubfoot [9,45-47]. However, other studies found no
association [48-52]. We did not use primiparity as a constraint predictor because almost half of all women
fall into that category and would have weakened the sensitivity of any particular constraint. For example, no
correlation has been observed for obesity since primiparity was added to the concept of ‘constraint.’ Clubfoot
is well known to recur in some obese families [45], And studies suggest that clubfoot cases had a relative
first degree affected [9,45,52-54].
Role of Socio-Economic Factors in Occurence of Clubfoot
Socio-demographic factors in clubfoot have not been consistently related; however, still they have a specific
impact on the child and mother both. Hence, we may consider them also as a sub-prominent factor for
clubfoot.
Level of education positively associated with clubfoot [9,45-47], however, other studies found no association
[48-52], as we observed. Further, the combined family income and its stability source have both direct as
well as indirect impact on clubfoot [9,47-49], by deciding the means of basic requirements for pregnant
women and infant both such as food, water, shelter, hygiene and early age medication etc [9,47-49].
We analyzed the status of cohabitation as a further measure of socioeconomic status in comparison to a
higher risk of unmarried mothers in a UK study, and it was also not associated with clubfoot [45]. Two
studies have identified a higher risk of clubfoot in White mother’s offspring compared to non-White
mothers [9,46], which was not substantiated in four other studies [47,49,50,52].
Proposed ANC Parameters Associated With Clubfoot
In at least some cases, clubfoot was accused of arising from fetal constraint and also thought to be associated
with ANC managements, including gestational age and medication also.
Based on clinical findings of cases involving oligohydramnios, uterine bicornuate, breech presentations,
and multiple births, i.e. females with no prior births are at increased risk of clubfoot. Past epidemiological
studies have, however, found contradictory findings, with positive, inverse and null associations [47-49,55].
Increased clubfoot risks associated with breech delivery and uterus bicornuate and 60 to 80% increased
risks for oligohydramnios and multiple births, in favour of a framework for limiting a minority of cases
[47-49,55]. The relation between clubfoot and primiparity which is regularly observed [45-52]. Was raised
as evidence to support fetal pathogenesis, on the premise that after the first birth, by holding the fetus, the
intrauterine area is extended [45,49].
Another potential pathogenetic mechanism for clubfoot is the vascular disturbance, leading to increased risk
of clubfoot in women with early gestational amniocentesis [55,56] Or is it exposed to abortive misoprostol
[57,58]. In a few studies, we found that if there is a 16 Weeks of gestation, amniocentesis, CVS, or the fetal
loss of a twin or triplet to be vascular disturbance markers. Such factors were correlated with an increased
risk of clubfoot 5.6, 2.2, and 4.1 times respectively, Supporting potential vascular disruption pathogenesis
[55-58].
Proposed Outcomes of Prenatally Diagnosed Clubfoot
Based on more systemic or neurological developmental abnormalities, it is suggested that the Prenatally
diagnosed isolated clubfoot cases will postnatally have complex clubfoot [26,27]. While advising women
on isolated clubfoot prenatally diagnosed, it is necessary to reassure them that approximately 10 percent of
people would have a normal foot or foot deformity that needs minimal treatment [26,33]. Clubfoot may
be diagnosed prenatally on a thorough ultrasonography scan performed in the second or third trimester by
visualizing Tibia and fibula in the same longitudinal plane as the foot’s lateral portion [47-49].
Conclusion With Future Perspectives
Clubfoot is one of the most prevalent birth defects in the musculoskeletal system; its etiology and
controversies related to optimal treatment strategies are still unknown. The aim of the study was to update
the latest progress in the awareness of the genetic and environmental etiology of clubfoot and explore the
future research required for this disorder to achieve a genetic classification scheme. The objective was also
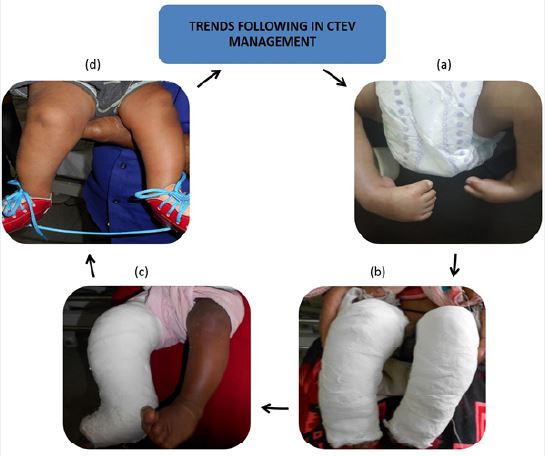
to examine the development of clubfoot management and clarify How it revolutionized the Ponseti process
childcare worldwide (Figure 4). Although current methods of treatment seem to be beneficial in maximum
cases, regardless of heir etiology, co-morbidity risks, i.e. hip dysplasia. Future genome associative research will provide an uneven alternative in the Identifying Clubfoot susceptibilities, and Large Samples Using
Identify the susceptibility of both major and minor genes where present. Individualized interventions based
on etiology may also lead to decreased use of braces if etiology or genetic profile correlates with risk of
relapse. The primary aim of treatment is to deliver a fully functional, painless foot for long-term correction.
In order to achieve this, more progressive research-based studies may need to combine approaches which
apply the strengths of different methods.
Acknowledgements
This study was supported by Department of Paediatric Orthopaedics & Orthopaedic Surgery in collaboration
with Department of Paediatrics and Department of Biochemistry, King George’s Medical University,
Lucknow.
Conflicts of Interest
The authors declare that they have no competing interests in this article.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!