Biography
Interests
Adedire Ayodeji1*, Jegede Vincent2, Obadire Florence3, Adedire Samuel4 & Oluwatosin Oluseyi2
1Department of Animal Science, Osun state University, Osogbo, Nigeria
2Department of Animal Nutrition, Federal University of Agriculture, Abeokuta, Nigeria
3Department Animal Science, Federal University, Dutse
4Department of Microbiology, Obafemi Awolowo University, Ile - ife, Nigeria
*Correspondence to: Dr. Adedire Ayodeji, Department of Animal Science, Osun state University, Osogbo, Nigeria.
Copyright © 2020 Dr. Adedire Ayodeji, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Agro-industrial bye-products (AIBPs) such as cowpea husk (CH) has been considered by researchers
to be an alternative feedstuff for livestock animals especially the ruminants and pseudo-ruminants
animals. However, the limitation to efficient utilization of these AIBPs is high level of lignocellulose
fibre which can be treated with enzymes from micro-organisms (especially fungi) through solid
state fermentation. Hence this study was conducted to assess the proximate composition of CH
fermented with single and mixed cultures of selected fungi over a period to determine the safest
day of fermentation for livestock use. Cowpea husks (CH) were collected from designated centres,
milled (1.0mm sieve) and degraded through anaerobic fermentation by single and mixed cultures
of selected fungi as follows: Aspergillus niger (ASP), Rhizopus oligosporus (RHZ), Trichoderma reesei
(TRI), A. niger +R. oligosporus (ARH), A. Niger + T. reesei (ATR) and T. reesei + R. oligosporus (TRH). The CH were allowed to ferment for ten (10) days and triplicate samples were taken from each
treatment every twenty-four (24) hour to analyze for proximate composition. The changes in
proximate composition for each was monitored. The results revealed that the dry matter content
of the fermented products were similar among the treatments, Crude protein of the fermented
crop residues were increasing with the duration of fermentation Crude fibre contents of the
fermented crop residues were shown to be reducing with the duration of fermentation Soluble
fibre fractions (neutral detergent fibre and hemicelluloses) were shown to be increasing with the
duration of fermentation Ether extract, ash contents, calcium were enhanced with longer duration of
fermentation It was observed that at the end of five days of fermentation, different microorganisms
other than those used as starter culture, were growing in the medium. It can be concluded that
fermentation of cowpea husk using mixed cultures of fungi enhanced the protein and soluble fibre
content of the residue. Also, fermentation up to five days is safe for cowpea husk intending to be
used as livestock feedstuff.
Abbreviations
AIBPs, CH. RHZ, TRI, ARH, TRH, SSF, SCP. CMC, MRVP, CP, EE, CF, NDF, ADF, ADL. NFC,
AOAC, NaOH, NaCl, UCH, MC, DM.
Introduction
Huge tonnage of agricultural wastes and agro industrial products (AIBPs) are generated annually all over
the world. From the production, processing and even consumption, there are great varieties of left over,
which creates problems of disposal. Sadly, much of these are disposed of by biomass burning, which is not
restricted to developing countries alone but is considered a global phenomenon, thus contributing to air
pollution and global warming. Use of crop residues to substitute concentrates may be an alternative means
of reducing high cost of production associated with all concentrate feeding systems in livestock production.
It has been emphasized that up to 40 - 50% of these AIBPs can clearly be utilized for animal feed [1,2]. A
preliminary study by Adeloye (1994) [3] indicated that cowpea husk is well accepted by goats. The author
reported that the increasing level of cowpea husk enhanced the intake of dry matter. Doma et al. (1999)
[4] concluded after assessing the performance of rabbits on cowpea husk and maize cobs that cowpea husk
can be incorporated into rabbits’ diet up to 40% while maize cobs can be up to 20%. Adedire et al. (2012)
[5] also concluded that cowpea husks and maize cobs are potential feed stuff for rabbit rations. utilization
as animal feed and recycling may be a better way off disposing AIBPs to ensure environmental protection.
However, the limitation to utilization of these AIBPs as feedstuff by livestock is high level of lignocellulose
fibre which can be treated by enzymes from microrganisms through solid state fermentation.
Solid state fermentation is an alternative process to produce fungal microbial enzymes using lignocellulosic materials from agricultural waste. These enzymes can be utilized to improve the quality of the lignocellulosic fibre and also save feeding cost. Agro industrial residues are generally considered the best substrate for solid state fermentation. SSF technology could be used to expand the range of feed ingredients available due to the ability of the process to improve digestibility of high fibre materials [6]. SSF technology offers an opportunity to produce a unique enzyme complex produce by various non-pathogenic fungi which can also serve as single cell protein. Promise in exploring microorganisms for single cell protein (SCP) production have been reviewed [7,8]. Therefore, the direct inclusion of non-pathogenic microbes in SSF technology will degrade the fibre and enhance the protein content of the feed. However, the use of mixed cultures has been known to degrade better than single culture [9], reason been that they tend to secret consortium of enzyme [10].
This study was conducted to assess the proximate composition of cowpea husk fermented by single and mixed cultures of fungi over a period to determine the safest days of fermentation for livestock use.
Materials and Methods
The materials used in this study were cowpea husk, Aspergillus niger, Rhizopus oligosporus, Trichoderma reesei. Aspergillus niger and Rhizopus oligosporus were collected from cultures bank of the department of Biological sciences, Wesley University of Science and Technology Ondo, Nigeria while Trichoderma reesei was collected from department of Microbiology, Obafemi Awolowo University Ile-ife, Nigeria. Equipment and utensils used are soxhlet apparatus, beakers petri dishes, muffle furnace, oven, autoclave, fume cupboard, inoculating chamber, microscope, durham tubes, haemocytometer, Atomic Absorption Spectrophotometer (AAS), Electrophotometer and bursen burner. Chemicals and reagents used are Carboxyl Methyl Cellulose (CMC), culture media, peptone water, violet stain, iodine, carbon fuchcin, hydrogen peroxide, MRVP solutions, Kovac’s, Griess-Hosvay’s and Koser’s reagent
The selected fungi used for fermentation as single culture were Aspergillus niger (ASP), Rhizopus oligosporus (RHS) and Trichoderma ressei (TRI) while A. niger + R. oligosporus (ARH), A. niger + T ressei (ATR) and T. ressei + R. oligosporus (TRH) were used as mixed cultures. Spores solution of each culture was generated by mechanical washing and the number of spores/ml was determined using haemocytometer. Cowpea husks were collected from designated centres, sun dried (≤90%DM) and then milled into 3.5mm size particles. Two hundred gram of the crop residues was measured into six different beakers each. Each beaker of the crop residue was inoculated with 20mls of spore (10-6) solutions as starter culture and labeled accordingly; ASP, RHZ, TRI, ARH, ATR, and TRH. The fermentation media were moistened at 230mls/100g [5] and mixed thoroughly for even distribution of the fungi spores. The crop residues were allowed to ferment anaerobically at 26oC for 10 days. Samples were collected at 24hours intervals and then analyzed for the proximate composition and fibre fractions.
Triplicate samples of fermented, unfermented cowpea husk were analyzed to determine crude protein (CP), ether extract (EE), crude fibre (CF) and ash on dry matter basis according to A.O.A.C, (2000) [11] methods. Neutral detergent fibre (NDF), acid detergent fibre (ADF) and acid detergent lignin (ADL) were determined according to the procedure described by Van Soest et al. (1991) [12]. Hemicelluloses was calculated as the difference between NDF and ADF, cellulose as difference between ADF and ADL and Non Fibrous Carbohydrates (NFC) using the equation NFC=100-(CP+EE+ASH+NDF) according to Calsamiglia et al. (1995) [13]. Calcium was determined using Atomic Absorption spectrophotometer according to the procedure of Perkin-Elmer (1968) [14] while phosphorus was determined colorimetrically using electrophotometer according to the procedure of A.O.A.C (2000) [11].
The results of the proximate composition, specifically crude protein and crude fibre content, were used to determine the optimal day of fermentation for each culture (treatment). The optimal day for each culture was recorded to be the day that the fermented crop residue had highest crude protein and lowest crude fibre content. Cowpea husks were fermented by each culture and their mixture until the obtained optimal day
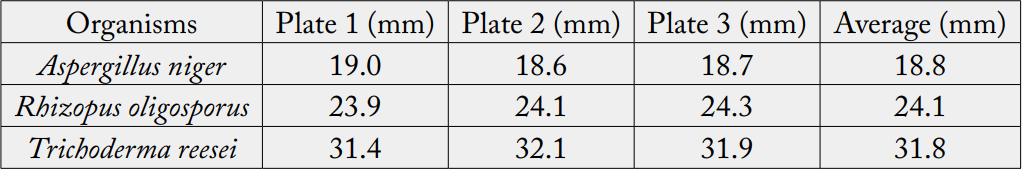
Assessment of Cellulase Produced by Experimental Fungi
The cellulose activity was determined by streaking the fungal cultures individually on carboxyl methyl
cellulose agar plates and incubated at 28o
C. After 5 days of growth the zone was identified around the culture
by treating the plate with Congo red and NaOH. After incubation, the plates were flooded with an aqueous
solution of Congo red (1% Congo red in distilled water) and shaken at 50 rpm for 15min on a shaker. The
Congo red solution was then poured off; plates were further flooded with NaCl and shaken again at 50
rpm for 15min. The fungal growth was stopped by flooding the CMC agar plates with HCI which changes
the dye colour to blue violet and further inhibited enzymatic activity. Unstained areas indicate where the
CMC has been broken down to b 1-4 glucans. The diameter of the clear zone was measured to provide
a quantitative comparison of cellulolytic activity, clearance around growth of isolate represents cellulose
production. The diameter zone of clearance was measured at three different locations and means of triplicate
determination was used to represent cellulase activity of the organism
Results and Discussion
Cellulase activities of Aspergillus niger, Rhizopus oligosporus and Trichoderma reesei screened on Carboxymethyl
cellulase agar is presented on table 1. This result confirmed that these fungi, Trichoderma sp, Aspergillus sp
[15-17] and Rhizopus sp (Gautam et al., 2012) are cellulase producers. Trichoderma sp gave higher yield of
the enzyme than Aspergillus by the interpretation of zone clearance and it is expected that Trichoderma sp
will degrade the substrate more than Rhizopus sp and Aspergillus sp. This work agrees with Sathyavrathan
and Krithika (2013) [18] who reported higher cellulase activity for Trichoderma sp as against Aspergillus
sp, however these results is contrary to Lee et al. (2011) [19] who obtained higher enzyme activity for
Aspergillus sp as against Trichoderma sp. This may be due to the changes in media composition where Lee et
al. (2011) [19] had used sugarcane bagasse and palm cake as substrates.
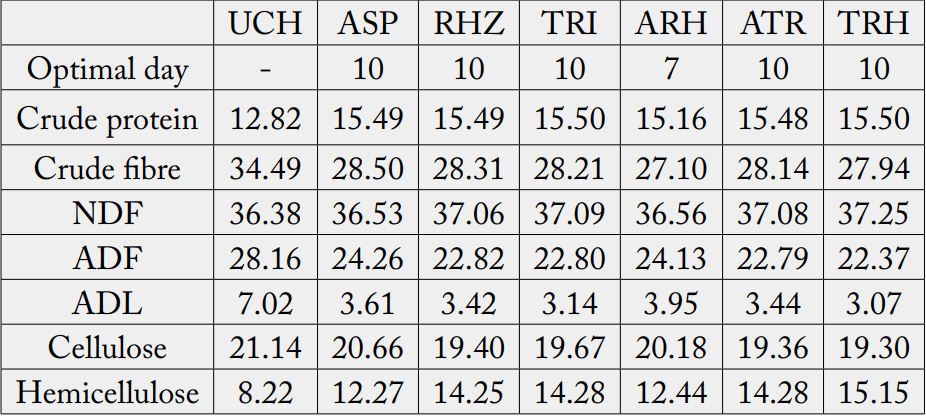
Changes in nutrients during cowpea husk fermentation with the single and mixed cultures of selected fungi for ten (10) days is represented on figure 1 - 6 while the Summaries of the Chemical composition of respective optimal day of fermented cowpea husk is presented on table 2. The results of fermentation of cowpea husk revealed that the dry matter content of the fermented products was similar among the treatments, this suggested that the treatment did not exert any effect on the dry matter. The similarity may be due to methodology used in preparing the fermented samples, they were dried to constant moisture content of 3-5% MC (i.e. 95-97%DM) to terminate the fermentation processes.
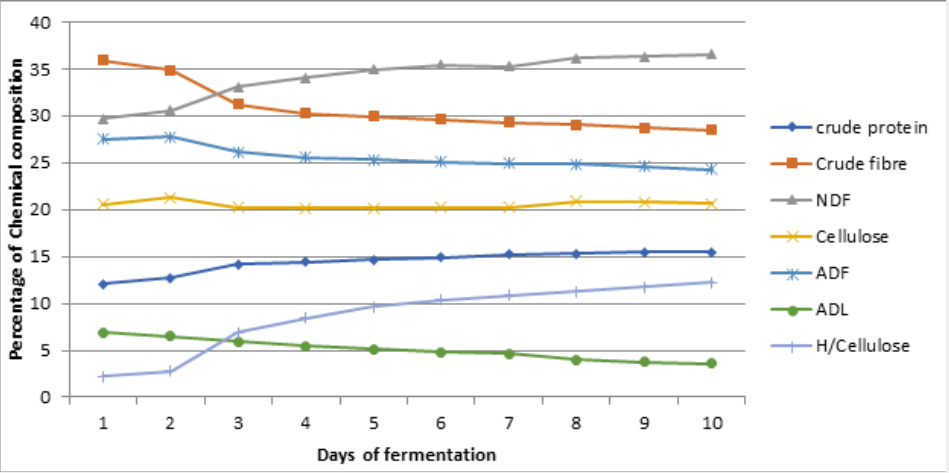
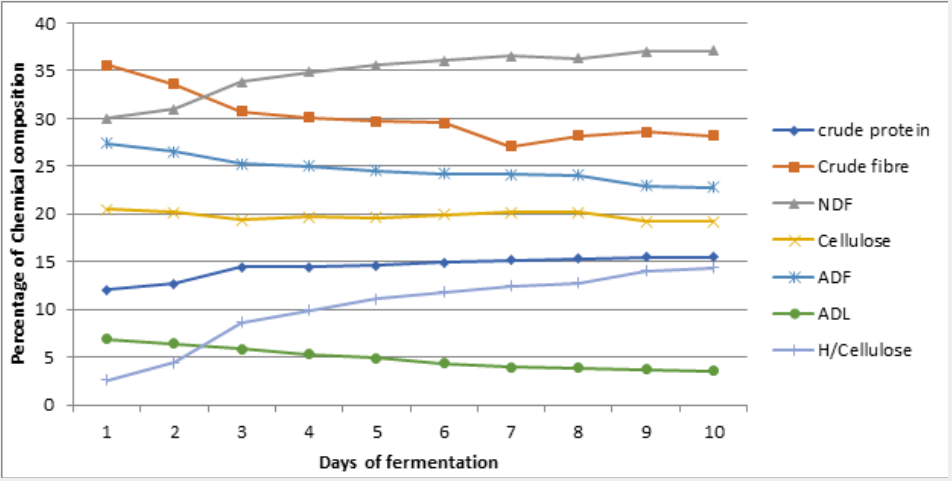
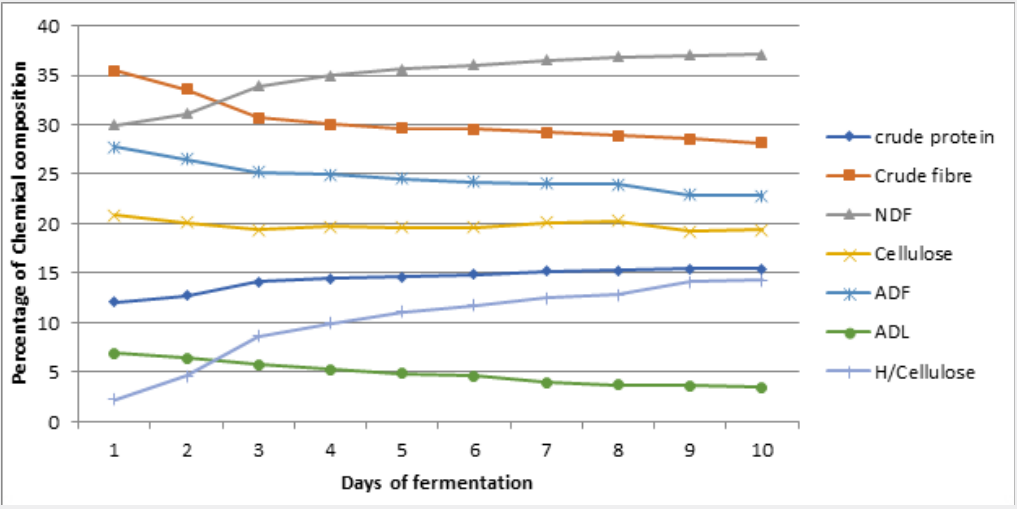
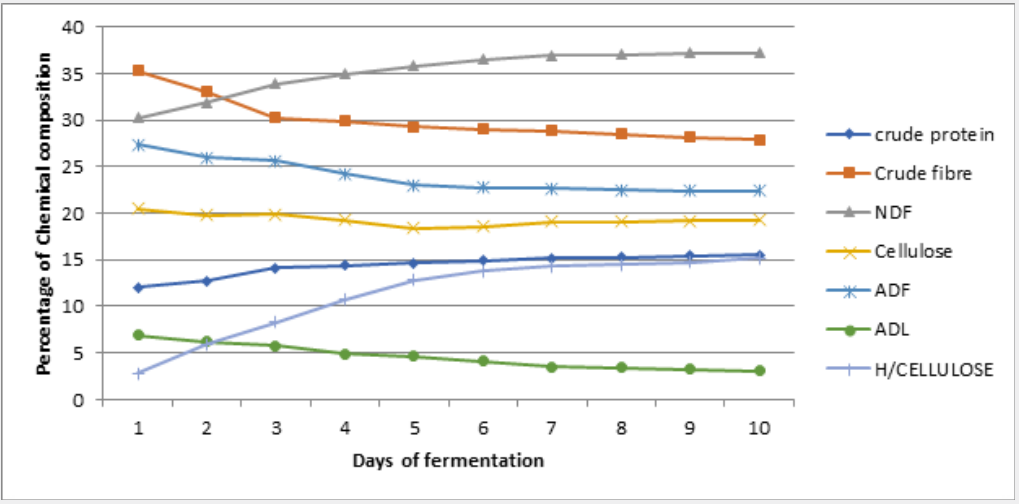
Note: UCH = Unfermented cowpea husk, ASP = Aspergillus niger, RHZ = Rhizopus oligosporus, TRI = Trichoderma ressei, ARH = A. niger + R. oligosporus, TR = A.niger + T.ressei, TRH = T.ressei+ R.oligosporus
Crude protein of the fermented crop residues was increasing with the duration of fermentation. After day 6 of fermentation of cowpea husk, crude protein content was increasing at a reducing rate. This result agreed with the report of Tehreema et al. (2010) [20], who reported an increase in protein content when Rhizopus oligosporus was used in fermenting soy bean to produced tempeh. Also with Oduguwa et al. (2008) [8] who reported an increase in protein when R. oligosporus was used as a single culture to ferment maize cob and cowpea husk. Iyayi and Aderolu (2004) [21] also reported an increase in protein content when corn bran was fermented with Trichoderma in a solid-state fermentation for 14 days. Charlotte (2002) [22] reported a similar result in which R. oligosporus along with Trichoderma hazarium and Aspergillus niger were used as mixed cultures to ferment pulp of sweet potatoes. The increase may be due to increase in population of fungi used in fermenting the samples and by implication the proteineous hyphae that make up the structure of the fungi. These micro-organisms are single cell proteins [7,8], therefore their multiplication will enhance protein content as they degrade the fibre. Hence, it may be suggested that the microbial protein contributed to a larger extent in the increase in protein content of the fermented products, and also the hydrolytic activities of the crude enzymes produced during the process can not be neglected [23]. Also, fermentation has been shown to release lock up nutrients in substrate and make it available for utilization [24,25].
Crude fibre contents of the fermented crop residues were shown to be reducing with the duration of fermentation. This is in accordance with the result reported by Belewu (2003) [26] when the lignocellulose fibre of some crop residues were degraded with selected fungi. Fermentation generally reduced crude fibre content in crop residue especially when fermented with fungi [8,27] because they possess ability to produce cellulase that degrade complex carbohydrates of lignocellulosic substrate to produce cellulose, hemicelluloses and may be soluble sugars. Therefore, the fungi used for fermentation in this study may have caused a reduction in fibre content. Also, increment in rate of fibre reduction with longer period of fermentation may be an indication that as the microbes increased in population over the period, they produced more cellulase to degrade the fibre content. It has also been reported by Martinez et al. (2008) [6] that fermented products had lower percentage of lignocellulosic fibre and more soluble fibre fractions when fermented with fungi, so the results obtained for crude fibre were not unexpected.
The result for the crude fibre of fermented products revealed that combination of Trichoderma reesi and Rhizopus oligosporus (TRH) were able to degrade fibre better than other treatments. This may be suggesting that enzyme activities of both T. reesei and R. oligosporus were better than the other treatments and combination seems to be synergistic and complimentary. It has been reported by Kumar et al. (2008) [28] that T. reesei had capacity to produce large amount of cellulase and hemicellulase with distinctly different activities which synergistically deploymerize cellulose. R. oligosporus has also been shown to increase the total soluble solids in fermentation [6,8].
Soluble fibre fractions (neutral detergent fibre and hemicelluloses) were shown to be increasing with the duration of fermentation. This is an indication that the lignocellulose fibre and cellulose in the crop residues were degraded during fermentation. Earlier works by Ofuya and Nwajiuiba (1990) [29] showed successful degradation of cassava peel by Rhizopus sp, the authors reported that over a quarter of the original cellulose content of the substrate were lost in solid state fermentation. It is also indicative of the fact that the fungi were able to act on the lignin (i.e. the cell wall constituents) that is a limiting factor in the use of crop residues. TRH had the highest values for these soluble fibre fractions. This may imply that the enzyme produced by TRH were able to degrade the lignocellulose fibre more than other treatments, thereby decreasing the crude fibre and the insoluble fibre fraction (Acid detergent fibre, Acid detergent lignin and cellulose) and increasing the soluble fibre fractions.
Ether extract, ash contents, calcium were enhanced with longer duration of fermentation. This observation may be attributed to the additional fat and body mass residues of the increasing microorganisms present in the substrates [25]. It is also likely that some nutrients especially minerals might be tied up by fibre which when broken down during fermentation could be made available [24] as Phosphorus was observed to be increasing with reduction in crude fibre content of the fermented products. This observation supports the fact that crude fibre binds phosphorus in feed stuffs [30] therefore as the fibre solubilized, the phosphorus is released [31]. The results revealed that non-fibrous carbohydrate (NFC) contents were reducing with longer duration of fermentation. This reduction may be linked with the activities of the microbes, that is, utilization of soluble portion of the carbohydrates for their growth and other energy demanding activities.
The optimal day of individual culture was similar. In this study, level of crude protein and crude fibre were used as criteria for determining the optimal performance of each culture. Optimal performance of any culture depends on the type of substrate [29]. The optimal day of RHZ when cultured on cowpea husk was 10 days, ASP also had similar optimal days. Meanwhile, Balogopalan (1996) [32] reported optimal day for Rhizopus sp when cultured on cassava peels to be 12 days. Chinedu et al., (2011) [33] reported 36 hours for Aspergillus niger when cultured on sugar cane pulp.
It was observed that at the end of five days of fermentation, different microorganisms other than those used as starter culture, were growing in the medium. This could be as a result of the breakdown of the cellulose to intermediate products such as hemicelluloses, glucose among others, in the fermentation medium which may have serve as substrate for the growth of other microorganisms that became ‘contaminants’ in these medium. It was well documented that the bacteria and the fungi that were isolated from these media (Bacillus sp, Pseudomonas sp, Serratia sp) are known to degrade cellulose and hemicelluloses [19,34]. They are also known to produce glucose which is one of the products of cellulose fermentation. Saccharomyces cerevisea was also isolated from the medium probably because of the presence of glucose which S. cerevisae used as substrate by converting it to ethanol [35].
Furthermore, the presence of easily utilizable sugars such as glucose has been reported to suppress production of enzymes through the action of catabolite repression [36]. Also, the activities of cellulolytic microbes are thought to be inhibited by ethanol as a result of end products inhibition of glycolytic enzymes and damage to cell membrane. The activities of these unwanted microorganisms may result in production of by - products, metabolites or mycotoxin which may not be desirable for the fermentation process thereby indicating a conveniently safe point to terminate the fermentation at day four.
It can be concluded that fermentation of cowpea husk using mixed cultures of fungi (especially Trichoderma reesi and Rhizopus oligosporus) enhanced the protein and soluble fibre content of the residue better than their respective single culture. Also, fermentation up to five days is safe for cowpea husk intending to be used as livestock feedstuff.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!