Biography
Interests
Ayda Khalifa, A. & Mutaman Kehail, A. A.*
Department of Food Technology, University of Gezira, Sudan
*Correspondence to: Dr. Mutaman Kehail, A. A., Department of Food Technology, University of Gezira, Sudan.
Copyright © 2019 Dr. Mutaman Kehail, A. A., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Natural products (e.g. Garlic bulb and Ginger rhizome) has been used in food production and in traditional medicine. The objective of the study was to run the GC-MS and phytochemical screening tests for Garlic (Allium sativum) bulbs and Ginger ((Zingiber officinale)) rhizome. The samples of the Garlic and Ginger were purchased from the local market, Wad Medani City, Gezira State, Sudan. The qualitative phytochemical screening was run in University of Gezira, Sudan, following the standard methods, whereas, GC-MS analysis was carried out at Forensic Laboratories Directorate, Khartoum State, Sudan. The results showed that, the GC-MS database identified 7 different compounds from ginger rhizome, and only 4 different compounds from the garlic bulbs. The qualitative phytochemical screening of garlic bulb detected the presence of saponins, flavonoids, glycoside, sterols and tannins, whereas that of ginger rhizome contained the same classes in addition to alkaloids. This work recommends studying both garlic bulb and ginger rhizome as food supplemented raw materials as well as a natural materials for controlling microbes.
Introduction
Garlic (Allium sativum) is a species in the onion genus, Allium. Garlic is suggested to be originated in the
regions of Central Asia and northeastern Iran. It has long been used worldwide [1], with a history of several thousand years of human trading activities. It was known to most ancient civilizations, and has been used
both in food processing and as a constituents in some traditional medicine preparations.
Garlic is usually used in foods because of its pungent flavor either raw or cooked. The garlic bulb is the only used part of the plant in most countries, and it is normally divided into fleshy sections (cloves). Garlic cloves have a characteristic flavor that considerably changed during cooking [2].
Ginger (Zingiber officinale L.) is a flowering plant belong to family Zingiberaceae. Ginger root or rhizome, is worldwide used in food processing as a spice and as a constituent in traditional medicine [3]. It is a herbaceous perennial plant, which grows untrue stems bearing the leaves. The inflorescences consisted of pale yellow and purple flowers that arises on separate shoots directly from the rhizome [4].
Ginger is native to Southeast Asia Islands and it was suggested to be domesticated at first by the Austronesians, and it was one of the oldest spices exported from Asia to ancient Greeks and Romans in Europe [5].
Ginger usually produces a hot taste and fragrant flavor [5]. Young fresh rhizomes are usually juicy and fleshy, but with mild taste. The volatile oils of ginger rhizome that compose 1-3% of the fresh weight, were responsible for the characteristic fragrance and flavor [6].
Gas chromatography-mass spectrometry (GS-MC) was used to analyze and compare the volatile components in the extracts. The chemical constituents revealed from the GC-MS analysis along with their retention time, base peak, molecular weight, molecular formula and compound names in addition to their figures were presented according to the library used to identify compounds [7].
Materials and Methods
The samples of Ginger (Z. officinale) rhizomes and garlic (A. sativum) bulbs were brought from the local
market of Wad Medani city, Gezira State, Sudan.
The selected products were first cleaned manually and then let to dry at room temperature away from direct sunlight. The selected samples were then crushed to fine granules. Each powder was extracted with 99% chloroform for 48 hours using cooled extract (5g plant powder dissolved in 25ml chloroform). Each extract was filtered to separate solid parts from chloroform-soluble part (CSP) under reduced pressure. The extract (CSP) was stored in refrigerator to prevent evaporation until running the GC-MS analysis.
The apolar parts of Ginger (Z. officinale) rhizomes and garlic (A. sativum) bulbs were analyzed using GCMS
technique. GC-MS analysis was carried out at Forensic Laboratories Directorate, Khartoum State,
Sudan. The chloroform-soluble part (CSP) was subjected to this test unlike the ethanol extracts.
In general, compound identifications depends on the database of National Institute Standard and Technology (NIST) interpreted on the GC-MS device. During fragmentation, and by using NIST library database, the unknown components were compared automatically with those stored in the database.
Phytochemical screening for the presence of the main classes in the selected samples were done according
to Yusha’u et al., (2009) [8].
In a test tube, to 3ml of each extract, 2 drops of Dragendoff ’s reagent was added. The precipitation of turbid
orange red complex denoted the presence alkaloids.
In a test tube, to about 4mg/ml of each extract a piece of magnesium ribbon was added then a drop of
concentrated HCl was wisely added. The presence of flavonones was confirmed when the orange colour
changed to red; while the presence of flavonoids was indicated by the red to crimson changes.
In a test tube, to about 1ml of the filtrate, 10ml of 50% H2SO4 was added. The mixtures was heated for 15
minutes then 10ml of Fehling’s solution was added and boiled. The presence of glycosides was indicated by
brick red precipitate.
In a test-tube, to about 0.5g of each powdered plant materials, 5ml of distilled water was added and the testtube
was shaken vigorously for 2 minutes. The presence of saponins was indicated by the formation of froth
that lasted for about 15 minutes.
In a test tube, 2ml of each extracts were evaporated to dryness. The residues were dissolved in acetic
anhydride then by addition of chloroform. Concentrated H2SO4 was gently added. The presence of steroids
was confirmed by formation of brown ring at the mid-layer between the liquids and the violet supernatant.
In a test tube, to 2ml of each extracts were diluted with distilled water, 3 drops of 5% ferric chloride (FeCl3)
solution was added. The appearance of green, black or blue colouration indicated the presence of tannins.
Results and Discussion
Concerning ginger rhizome, the GC-MS chromatogram draws 47 peaks, but database identified 7 different
compounds from their chloroform extract: Benzoguanimine (C9H9N5), Levomenthol (C10H20O), Anthracene
(C26H48), Diethyltoluamide (C12H17NO), Versalide (C18H26O), Isopropyl myristate (C17H34O2), and
Alpha Amyrenyl acetate (C32H52O2) as was shown in Table (1).
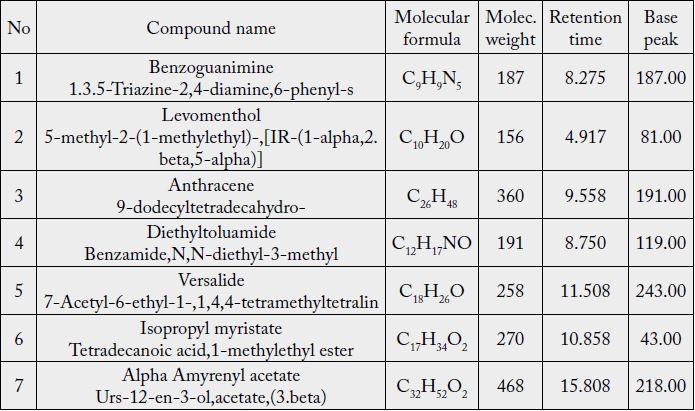
In similar study, the results showed that 140 components were separated and about 74 of them were tentatively identified [9].
In other similar study, the principal constituent in ginger oil was Zinziberene, a kind of sesquiterpenes hydrocarbon its content was higher in Chinese than Guinean ginger. It was found that, the β-sesquiphellandrene was higher in Chinese ginger compared to Guinean ginger. The low boiling point monoterpenes a-pinene, Zinziberenol, Linalool, Terpineol, Borneol, Eucalyptol, 1, 8-Cineole, Cubenol, Cadinol, β-Eudesmol and Nerolidol were more abundant and present in various proportions and they imparted aromas characteristic to the products [10].
GC/MS analysis of methanol extract of Zingiber officinale rhizomes revealed the existence of Zingiberene, followed by AR-curcumene, α-Bergamotene, Gingerol, Zingerone, β-esquiphellandrene, (Z)-β-Farnesene, Caryophyllene and c-Elemene [11].
The GC-MS identified only 4 different compounds from the chloroform extract of garlic bulbs: 1,2-Benzenedicarboxylic acid,butyl,2-ethylhexy ester (C20H30O4), Mononitromesitylene (C9H11NO2), Bisoflex (C24H38O4) and Lupenyl acetate (C32H52O2) as was shown in Table (2). The GC-MS identified only 4 different compounds from the chloroform extract of garlic bulbs: 1,2-Benzenedicarboxylic acid,butyl,2- ethylhexy ester (C20H30O4), Mononitromesitylene (C9H11NO2), Bisoflex (C24H38O4) and Lupenyl acetate (C32H52O2) as was shown in Table (2).
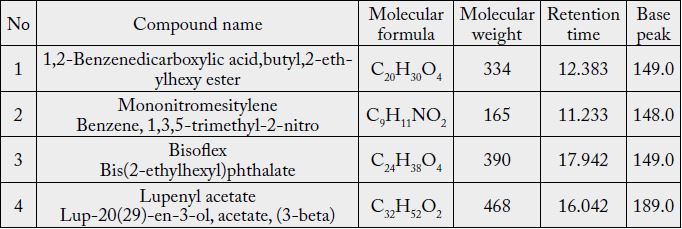
The active components of ethanol garlic extracts that compared through GC-MS analysis showed that, allyl trisulfide, 2-hydroxy-gamma-butyrolactone, 1 3-dihydroxyacetone dimer, propanoic acid, and 2-propone were detected in all extracts. These substances (Appendix, 1) are produced by the degradation of alline and have been reported to have beneficial effects, such as antioxidant and anticancer activities [7].
The qualitative phytochemical screening of Ginger rhizome and Garlic bulb were presented in Table (3). Ginger rhizome contained glycosides, flavonoids, saponins, sterols and alkaloids, as same as Garlic bulb (except in alkaloids which was not detected in garlic bulb). Tannins in both samples did not detected.
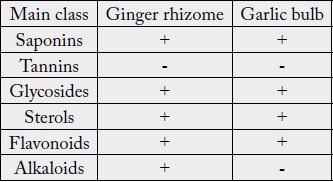
Phytochemicals usually give plants its characteristic colour, odor and flavour, and consider part of its internal defense mechanism against pests, parasites and pathogens [12]. Ali and Ibrahim (2019) [13] detected the presence of alkaloids in Garlic bulbs. Deresse (2010) [14] found that garlic extract exhibited activity against both gram negative and gram positive due to presence of some phytochemicals such saponins and tannins.
Conclusion
Based on the results of the present study, the GC-MS database identified 7 different compounds from
ginger rhizome chloroform extract, and only 4 different compounds from the garlic bulbs. phytochemical
constituents, proximate and minerals components of garlic bulb were determined. The qualitative phytochemical
screening of garlic bulb detected the presence of saponins, flavonoids, glycoside, sterols and tannins,
whereas that of ginger rhizome contained the same classes in addition to alkaloids.
Recommendations
Based on the findings of the present study, both garlic bulb and ginger rhizome should be studied as food
raw materials as well as a natural materials for controlling microbes.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!