Biography
Interests
Samar Fadl, M. & Tarek Madkour, M.*
Department of Chemistry, The American University in Cairo, AUC Avenue, Egypt
*Correspondence to: Dr. Tarek Madkour, M., Department of Chemistry, The American University in Cairo, AUC Avenue, Egypt.
Copyright © 2019 Dr. Tarek Madkour, M., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
In order to contribute to sustainable development, alternatives to fuel-based polymers must be employed. Poly (lactic acid) (PLA) is a common natural polymer that is currently used in food packaging applications but with limited applicability due to its poor mechanical behavior (high elastic modulus and low elongation at break). To overcome PLA weaknesses and to enhance its flexibility and applicability in food packaging while preserving its environmentally friendly nature, three natural plasticizers, namely polyethylene glycol (PEG), tri n-butyl citrate (TBC), and triacetin (TA), are employed in this study. Stress-strain measurements showed that PEG did not enhance the mechanical response of PLA. Strangely, increasing PEG content has resulted in worsening the mechanical response of PLA, which was attributed to the formation of hydrogen bonding between PEG hydroxyl groups and PLA carbonyl oxygens, which limited the extensibility of the chains and increased its stiffness. Different concentrations of TBC and TA plasticizers were far more efficient when compared to PEG ones. The energy values of PLA/ TBC and PLA/TA samples have shown significant increase over PLA/PEG samples. Quite interestingly, stress relaxation of 10% PLA/TA samples showed an anomaly with upturn in the sample modulus at higher elongation values indicating an increased toughness of the samples with strain. This unique behavior was attributed to the formation of small crystallites resulting from the ordering of the polymeric chains at higher strain values as was confirmed by DSC measurements.
Introduction
It was estimated that 280 million tons of plastics were produced globally in 2012 alone with the largest
contribution of plastic products is in the field of food packaging [1,2]. Although plastic products have many
desirable aspects such as low cost, lightweight, ease of processing, they possess many drawbacks. One of the
main disadvantages of plastic materials is their manufacturing from depleting resources [3,4]. Furthermore,
these fossil-based polymers are non-degradable materials and exert an incredible stress on the environment.
According to statistical studies, plastic pollutants accumulate in the environment as their degradation process
last from hundreds to thousands of years [5]. Not only traditional plastics are harmful to both the economy
and the environment but also to human health. Different types of additives are usually added to plastic
products to improve their properties. Nevertheless, there is an increasing doubt regarding the safety of these
additives, and there is a growing interest in studying the effect of additives leaching on human health [4].
It is, therefore, obvious that environmentally friendly alternatives other than traditional plastics such as
biodegradable PLA need to be employed in such applications as in food packaging [6]. It has been reported
that they have desirable features such as providing better food preservation as well as enhancing the shelf-life
for packaged food but unfortunately exhibit poor mechanical properties that limit their usage [7-9]. Different
approaches have been implemented to overcome these drawbacks including copolymerization, sampleing,
and plasticization [10,11]. Having low molecular weight that ranges from 300 to 600g mol-1 permits the
plasticizer to pervade the intermolecular voids between the polymer chains. As a result, the secondary forces
among polymer chains are minimized; this leads to a smoother chain motion and hence a reduced glass
transition temperature (Tg) of the investigated polymer. It was proved that Tg value is dependent on the
mobility of polymer chains. Currently, there is a growing trend towards replacing traditional phthalatebased
plasticizers with natural-based plasticizers made from biodegradable and/or renewable resources. For
food packaging applications, PLA has been plasticized with a wide variety of biodegradable plasticizers.
Plasticizers approved for food packaging applications must meet specific characteristics of nontoxicity and
no volatility during processing. They should also lack a tendency to migrate to the matrix surface and must
be miscible with the polymer. Lactide monomers were used to plasticize PLA with a remarkable increase in
the elongation at break. However, they were found to migrate to the surface of the polymer due to its low
molecular weight, which resulted in a stiffer PLA on the long term [12]. To overcome this problematic issue,
oligomeric plasticizers of relatively larger molecular weight have been investigated [13]. Different loads of
plasticizers with large molecular weights were also studied and it was reported that at higher loads, phase
separation may take place [14-16]. Other studies addressed the problematic issue of plasticizers migration
by grafting plasticizers to the surface of PLA using reactive extrusion techniques but this approach had a
declining effect on the softening of the polymeric matrix and didn’t contribute greatly to the improvement
of the mechanical response of the systems [17,18].
It is the purpose of this work to develop environmentally friendly biodegradable plasticized PLA materials with excellent mechanical characteristics for use in food packaging applications. The study will cover the use of different loads of a variety of natural plasticizers as to select the sample with the best stress-strain profile. The three plasticizers employed in this study were chosen based on their ability to impart flexibility to PLA, their compatibility, their low toxicity, and their “Generally Recognized as Safe” (GRAS) properties. The study is also extended to investigate the fundamental principles behind the peculiar behavior observed for PEG-plasticized and low concentration TA-plasticized PLA samples using the specific technique of stressrelaxation as to provide a clear understanding of the structure-property relationship between the polymer and the plasticizers.
Materials and Methods
Poly (lactic acid) (PLA) with a commercial name “Ingeo” grade 4043D, was purchased from NatureWorks
LLC, Minnetonka, USA. Ingeo 4043D had a density of 1.24g cc-1, a relative solution viscosity (RV) of 4.0
± 0.10, and a D-isomer level of 4.25 ± 0.55%. Plasticizers selected for this study were all chosen to be biocompatible
with PLA, have no known toxicity, and are classified as (GRAS) (Generally Recognized as Safe).
Polyethylene glycol (PEG) was purchased from Loba Chemie, Mumbai, India and having molecular weight
of about 400g mol-1. Tri-n-butyl citrate (TBC) and triacetin were supplied by Alfa Aesar, Karlsruhe, Germany.
Both plasticizers had purity of 99%. Dichloromethane (DCM) solvent was purchased from Sigma
Aldrich, Germany. All supplied materials were used as is without further purification.
Prior to mixing PLA with the plasticizers, PLA was vacuum dried at 40ºC and 600mbar for 5 hours. The
method adopted to fabricate plasticized PLA films was “solvent casting” technique. To prepare PLA/plasticizers
samples, 100g kg-1 PLA/DCM solutions were prepared. Three different concentrations (100g kg-1,
200g kg-1, and 300g kg-1) for each plasticizer (PEG, TBC, and TA) were added separately to an Erlenmeyer
flask with DCM solvent and allowed to stir for 10 minutes to ensure homogeneous mixing of the plasticizers
and the DCM. These concentrations were chosen to mimic the plasticizer concentrations typically used
in the industrial production of food packaging materials. The pre-dried PLA was then added to the plasticizer/
DCM solution and was left to stir for 15 hours until complete dissolution. The Erlenmeyer flasks were
sonicated for 30 minutes to eliminate air bubbles. The polymeric solutions were then cast on smooth, free of
scratches glass plates using a laboratory-designed applicator. The initial thickness of cast solution was 1mm
that decreased to about 120μm after complete evaporation. The cast films were kept in a closed environment
to avoid formation of air bubbles and/or deformation of films during evaporation that extended for 24 hours
at room temperature. The glass plates were then immersed in distilled water for 10 to 15 minutes to facilitate
peeling off the films. To eliminate residual solvent, which also acted as a plasticizer, films were further
vacuum dried at 20ºC and 600mbar for 24 hours.
The stress-strain isotherms for the various samples at room temperature were obtained from casted film
specimens. According to ASTM D882 method, samples free of pinholes and air bubbles with specific
length, width, and thickness of 9.0cm x 1.5cm x 0.0085cm, respectively, were cut from the PLA films to
evaluate the mechanical response of the samples and the influence of the addition of the plasticizers on the
mechanical behavior of the samples. The apparatus used for the stress-strain measurements is described
elsewhere [19]. Samples were held vertically between two clamps with the lower clamp being fixed while
the upper clamp was suspended from a strain gauge (Stautham model G1-16-350). A constant voltage DC
power supply (Hewlett Packard 6217) was used to supply approximately 14volts potential to the transducer
and was connected to DS1M12 digital oscilloscope & WaveForm generator for output data recording. The
transducer was frequently calibrated using a set of standard weights prior to use for every sample. Its output
was found to remain constant over the usual time span of an experiment. Prior to attaching the clamps to a
sample, two thin lines were drawn on it by means of permanent marker. The sample clamps were lined with
thin sheets of rubber to minimize premature rupture or slippage of the samples at the contact points. The
exact length of the thus-demarcated section of the sample was measured precisely at the desired temperature
by means of a cathetometer (Gaertner Scientific Corp., Model M940-303P, Precision 1 micron), and the
thickness and width of samples were determined with a micrometer. Three measurements each along the
thickness and width of the sample were taken, and the average cross-sectional area, A*, was determined.
The upper clamp was raised to a position giving the desired elongation of the strip in a step-wise fashion.
The distance between the two lines was measured with a cathetometer and recorded as the length L. The
ratio of L and Lₒ (initial length) presents the elongation (strain), α. The potential from the stress gauge
was calibrated in terms of Newtons (N). The stress relaxation measurements were made using a sequence
of increasing values of the elongation. The values of the elastic force σ were recorded at every strain step
for the duration of the initial 30 minutes of the relaxation experiment. The equilibrium elastic force, ƒ, was
noted after the force reading has become sensibly constant for at least 15 minutes and is used to calculate
the nominal force (ƒ*=ƒ/A). The elastic quantity of interest for the stress-relaxation investigation portion
was the reduced stress, [ƒ*], or modulus defined by: [19]
Three replicates of each sample were tested.
To assess the effect of plasticizers on the thermal behaviour of PLA matrix, differential scanning calorimetry
instrument (PerkinElmer PYRIS Diamond Autosampler, Waltham, Massachusetts, USA) was employed.
The investigated films were cut into small pieces, and around 17mg of each specimen were weighed into a
50μL aluminum pan and sealed with a cover using universal crimper press. Heating-cooling-heating cycles
were performed under nitrogen atmosphere. During the DSC experiment, samples were heated from room temperature to (200ºC) at a rate of 10ºC/min, where upon, the temperature was held at (200ºC) for 10
minutes to remove thermal history [20]. The samples were then cooled from (200ºC) to (-20ºC) at a rate of
10ºC/min and again held at (-20ºC) for 10 minutes. The next step was to reheat the samples from (- 20ºC)
to (200ºC) at a rate of 10ºC/min. The glass transition temperature (Tg), cold crystallization temperature (Tcc),
and melting temperature (Tm) were all determined from the second heating scan [20,21].
Results and Discussion
Stress-strain isotherms of neat PLA and plasticized PLA using three different concentrations of three
different plasticizers are represented in Figs. 1-4 by plotting the nominal force (stress), f*, versus the
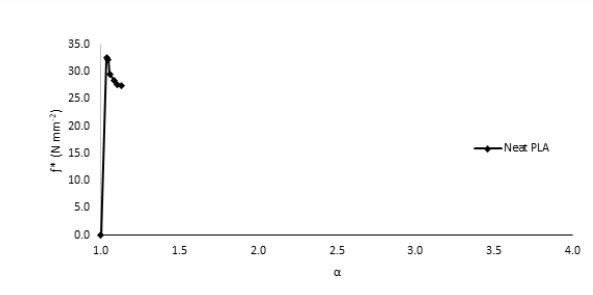
elongation (strain), α, for all the samples. The high modulus and very low tensile elongation of PLA is
clearly shown in Fig. 1. This behavior was attributed to the high glass transition of PLA (about 63°C). Below
Tg, PLA exhibits rigid behavior and low mobility that restrict its application to food packaging.
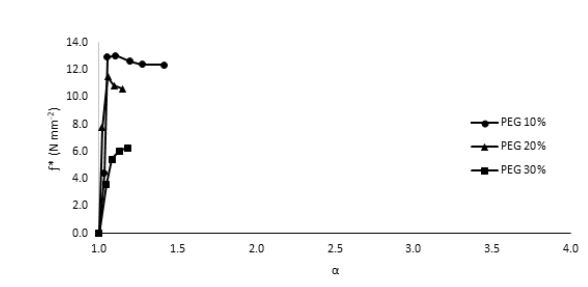
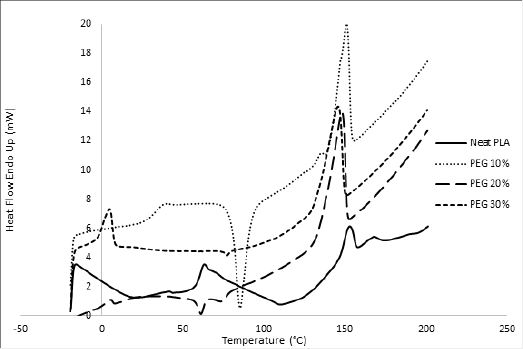
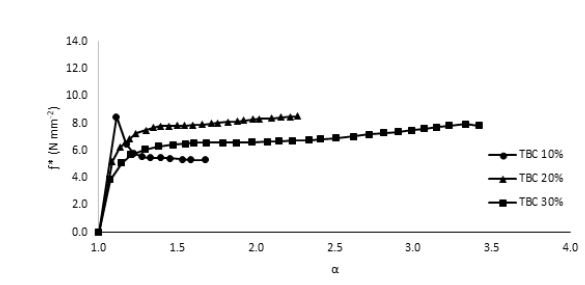
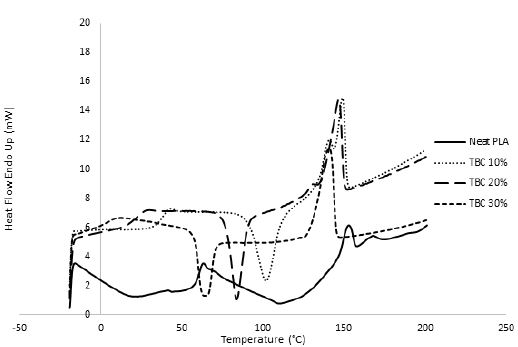
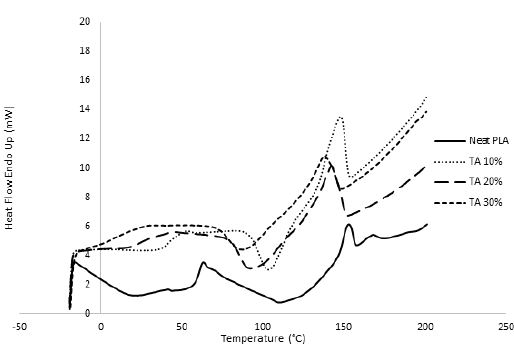
In order to overcome the stiffness of PLA, PEG plasticizer was added to PLA with three different concentrations, namely 10, 20, and 30% by weight. Fig. 2 showed that the elongation (α) has slightly increased on the expense of the elastic modulus, which showed in turn a significant decrease for all PEG plasticized samples. Interestingly, further increase in the amount of PEG has adversely reduced the elongation instead with an associated decrease in the nominal force as would be expected. This behavior could be attributed to the formation of small crystallites within the polymeric matrix upon mixing PLA with PEG as observed in the DSC measurements, Fig. 3. These crystallites acted as to restrict the motion of the ordered PLA chains and reduced its flexibility in response to the increase in PEG content and thus resulting in a greater stiffness of the plasticized samples at higher concentrations. The strong interactions between the hydroxyl groups of PEG molecules and the carbonyl groups of the PLA chains had also adversely affected the process of softening the PLA matrix leading to softer polymeric samples with the increase in the plasticizers content. This phenomenon has been reported in the literature as “antiplasticization effect” and can result in further increase in the rigidity of the polymeric matrix instead of enhancing its flexibility [22,23]. Table (1) compares the maximum elongation at break (α m), the maximum nominal force at break (ƒ*m) and the amount of energy required to break the sample or to reach maximum elongation for neat PLA and PLA/Plasticizer samples at different concentrations (Em). The table clearly shows that the maximum nominal force has significantly decreased by the addition of PEG with a slight increase in the maximum elongation at break. The low energy required to break neat PLA and PLA/PEG samples indicated the low resistance to applied stress due to the inability of the molecular chains to move freely. Unlike PEG plasticizer, isotherms of samples with the TBC plasticizer Fig. 4 demonstrated a significant increase in the elongation, which was associated with a decrease in the nominal force as expected. The increase in the flexibility of the chains in this case was attributed to the decrease in the glass transition temperature (Tg), which was demonstrated through DSC analysis, Fig. 5. Tg has decreased from about 63°C to 43°C for samples with 10% TBC and to even lower values for higher concentrations of TBC. TBC plasticizer has obviously increased the flexibility of PLA chains by creating free volumes within the PLA matrix as a result of the repulsive interactions between its molecules and the chains but on the expense of the nominal force of the samples. A remarkable increase in the energy required to break the PLA/TBC samples, Table 1, is attributed to the increased toughness of the samples resulting from the apparent increase in the elongation of the samples that facilitates the motion of the molecular chains as to resist the applied stress.
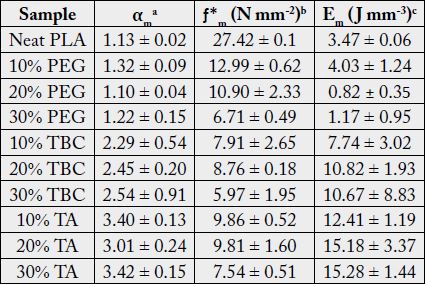
To render PLA suitable for food packaging applications, another plasticizer, TA, was used to modify its characteristics. Fig. 6 illustrates the influence of TA concentration on the mechanical properties of TAplasticized PLA. The elongation was shown to have increased by more than 165% for all the TA-plasticized samples. The plasticizing effect of TA was proved by DSC analysis, which demonstrated a remarkable decrease in Tg values by more than 10ºC with respect to that of neat PLA. Compared to PEG and TBC plasticizers utilized in this study, TA proved to be more compatible with PLA as was evident from both the mechanical and thermal behavior of the samples. This is possibly due to the structure similarity between TA and PLA. As shown in Table 1, the addition of TA to PLA has produced the highest maximum elongation at break among all other investigated plasticizers. In comparison to the other plasticizers, the maximum nominal force was maintained in spite of the increase in elongation. This is quite interesting since naturally the increase in the sample flexibility and therefore its elongation takes place on the expense of its nominal force (stress) unlike the case of TA-plasticized samples, which may be attributed to the high compatibility of TA with PLA matrix. Maintaining the high values of the nominal force, with an appreciable increase in the elongation has resulted in quite high values for the energy required to break the samples, Table 1, resulting in samples with the highest observed toughness.
To investigate further the basis behind this augmented toughness, alternative representation of the stressstrain data based on the Mooney-Rivlin equation:
where 2C1 and 2C2 are constants [19],
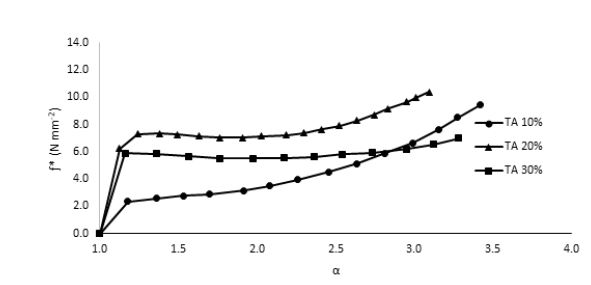
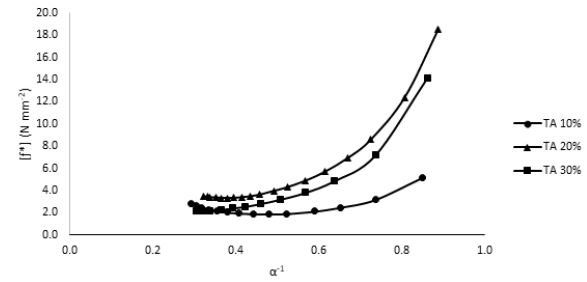
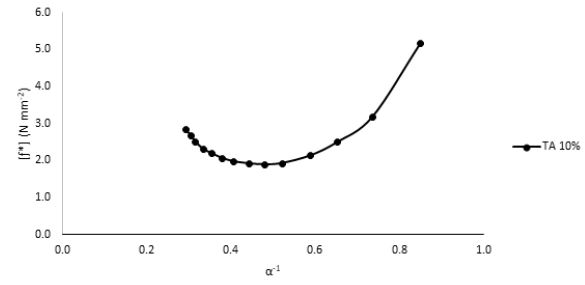
was implemented. According to the above relationship, the reduction in the sample modulus, [ƒ*], with the increase in elongation (or decrease in the reciprocal elongation, α-1) is expected. These stress relaxation curves showed normal behavior for both TA-plasticized samples 20% as well as 30% TA where the moduli showed a gradual decrease at higher elongation values, Fig. 7. This behavior is expected since at higher elongation values, the material experiences higher stress that tends to weaken the investigated sample until it reaches the break point. However, for 10% TA sample, an interesting behavior was observed, Fig. 8. Instead of decreasing continuously with the increase in the elongation values, the modulus demonstrated a unique upturn behavior, which implies that in response to applied stress, the polymeric chains started to strengthen further at high elongations instead of weakening. This improved toughness was observed only for the 10% TA/PLA samples. This interesting observation could be explained in terms of the “strain-induced crystallinity” effect, where at higher elongation values, the PLA polymeric chains experience some kind of order, which causes the formation of small crystallites within the polymeric matrix, which adds to the total strength of the sample [24]. This behavior is quite unique for 10% PLA/TA sample in this study. By further increasing the TA plasticizer content to 20% and 30%, the upturn disappears and both concentrations demonstrates normal behavior due to the dissolution of any formed crystallites in the presence of high concentration of the plasticizer, Fig. 9. Therefore, and due to this enhanced mechanical strength, adding 10% of TA to PLA samples is considered as the optimum concentration for these systems. Such performance of 10% TA/PLA is of a significant impact particularly in food packaging industry where high flexibility and resistance to tear at high elongations are highly required.
Conclusion
To provide the food packaging industry with an environmentally friendly biodegradable material with
optimum mechanical performance, different natural plasticizers of different concentrations were added to
poly (lactic acid) (PLA). Results obtained from the stress-strain measurements showed that PEG did not
enhance the mechanical properties due to the “antiplasticization” effect due to the formation of strong
hydrogen bonding network between the hydroxyl groups of the PEG molecules and the carbonyl groups on
PLA chains leading to polymeric chains with diminished flexibility. High crystallinity of PLA/PEG samples
as shown by DSC measurements accounted for the stiffness of the PLA prepared films. Alternatively, TBC
and TA plasticizers were far more efficient in plasticizing PLA when compared to PEG. This finding was also
confirmed by DSC analysis. Although both TBC- and TA-plasticized samples of different concentrations
showed a significant enhancement of the mechanical properties in terms of both the stress and strain of the
samples, only 10% TA/PLA samples showed an interesting anomaly where the stress has started to increase
at higher elongations. This interesting behavior has considerably increased the toughness of the PLA matrix
accompanied with much improved flexibility. This implied that the 10% TA/PLA sample would eventually
show high performance due to the much improved high energy required for breaking the samples. It is,
therefore, recommended that 10% TA-plasticized PLA be employed in food packaging applications where
high flexibility and toughness are both required. This would also serve the purpose of maintaining the
environment through incorporating biodegradable and GRAS packaging materials.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!