Biography
Interests
Solís Pacheco, J. R.1, Rodríguez Arreola, A.1, Gutiérrez Padilla, J. A.2,3, García Morales, E.2, Martínez Preciado, A. H.1, Castro Albarrán, J.1, Balcázar López, E.1, Cavazos Garduño, A.1 & Aguilar Uscanga, B. R.1*
1University Center of Exact Sciences and Engineering, University of Guadalajara, Blvd, Guadalajara, Jalisco,
Mexico
2O.P.D. Former Civil Hospital of Guadalajara “Fray Antonio Alcalde”, Division of Pediatrics, Neonatology service
/ Joint Accommodation, Guadalajara, Jalisco, Mexico
3University of Guadalajara. University Center of Health Sciences, Department of Human Reproduction Child
Growth and Development, Guadalajara Jalisco, Mexico
*Correspondence to: Dr. Aguilar Uscanga, B. R., University Center of Exact Sciences and Engineering, University of Guadalajara, Blvd, Guadalajara, Jalisco, Mexico.
Copyright © 2019 Dr. Aguilar Uscanga, B. R., et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
In Mexico and in the world, there is a need to feed to newborn infants, who for some reason their mothers cannot breastfeed them and due to this situation, Human Milk Banks (HMB) have been implemented using the pasteurization with subsequent freezing, as a method for its conservation. However, these methods disfavor the nutritional and physical-chemical quality of breast milk. Therefore, we have applied the spray drying after the process of pasteurization, as an alternative for the preservation of human milk. Spray drying was applied in human milk samples, at temperatures of 150 to 200ºC with a feed flow of 1 to 2mL/min. The milk powder collected was analyzed with respect to the nutritional content (proteins, lipids, sugars) and microbiological quality. The results showed that the pasteurization and spray drying processes do not affect the total content of proteins, lipids and carbohydrates in human milk. The microbiological quality, no growth of any type of microorganisms was observed in the samples after the treatments. However, the content of immunoglobulins decreases between 70 to 80%. We propose, that the process of spray drying of the human milk in combination with pasteurization, can be an important alternative for the Human Milk Banks to conservation of human milk.
Introduction
Human milk is a biologically active food that contains a mixture of nutrients and immunological
constituents that have potential properties for the modulation of the immune system. Breast milk has
numerous growth factors, which act on the gastro-intestinal tract, vascular, nervous and endocrine system
in newborns [1]. Among the components with biological properties are epithelial cells, immunoglobulins,
cytosines, chemokines, hormones, mucins, oligosaccharides, growth factors and antimicrobials [2]. Some
authors attribute the high bioavailability of human milk to the high amount of lactoferrin present, since
minerals such as calcium, magnesium, iron, copper and zinc that are bound to serum proteins, and the
protein membrane of the globule of fat [3].
Human milk has numerous substances, both humoral and cellular, that provide specific protection against infections. The most important evidence derives from numerous epidemiological studies, where morbidity and mortality from infectious diseases are greatest when children are fed formula milk with milk; and it increases when children fed with breast milk begin their weaning [4].
Breast milk transmits antibodies, contains all the immunoglobulins (A, G, E, M, D), being the most important due to its concentration and biological characteristics, IgA. It also contains lactoferrin, macrophages and mobile phagocytes with a large amount of lysozyme capable of synthesizing immunoglobulins [5].
Fresh breast milk can be stored at refrigeration temperature for 96 hours. After this period, it is not advisable to consume it since the total protein could decrease and the free fatty acids increase [6].
Pasteurization represents a conservation alternative practiced for many years in the field of food technology. The objective is to inactivate the thermos resistant microorganisms and the hepatitis and HIV virus, which is inactivated at 56ºC for 30 minutes. The applied temperature of 62.5ºC for 30 minutes and the rapid cooling to 5ºC, guarantees a high degree of inactivity of the virus and inactivation of 100% of the pathogenic microorganisms and 99.99% of the saprophyte microbiota [7]. After pasteurization, the breast milk should be frozen as soon as possible at -18ºC and stored in airtight containers for preservation. This milk can be kept for 4 months, however, in this period the nutrients of the milk are not guaranteed [8].
For milk to be consumed by infants, it must be thawed and refrigerated for use within 24 hours, but never refrozen. It is recommended that if you do not know the amount that the baby will take, it is advisable to use different containers to store the milk and feed by dose, in order not to waste the milk [6].
The Human Milk Banks generate economic and health benefits for families in México, since had feed a baby with breast milk reduces the days of stay and consequently the hospital expenses of premature newborns. Mainly families are benefited by avoiding the purchase of breast milk substitutes or “milk formulas”. In Mexico we have 19 Human Milk Banks [9], with a daily production of around 227.14mL of milk collected and pasteurized [10]. It is known by reference that 30% of this milk is discarded for quality reasons and as a protection of the health of newborns, either due to poor pasteurization or contamination of poor management [11,12].
The newspaper EL MILENIO public in November 2015 [13], that the HMB of the Hospital Materno Infantil López Mateos of the City of Guadalajara, Jalisco in México, that approximately 60 liters of human milk of a total of 200L collected are wasted a month, due to safety protocols that the hospital must comply with. Due to this problem, they have been increased of precautions for milk extraction and donor control, however did not eliminate the total contamination caused by microorganisms. This is a problem that all BLH have worldwide and unfortunately do nothing to solve this problem.
The Human Milk Banks (HMB), are provide limited to the needs of newborns with breastfeeding problems, due to at the control of donors and mainly of the process of pasteurization that leads to the freezing of milk. Since it is proven that these processes of conservation, together or separately, they can destroy of the nutritional and functional properties of human milk. Usually, the human milk is heat treated in the hospitals, particularly to destroy contaminating bacteria and viruses, but this treatment simultaneously reduces the content of some vitamins, enzymes, and immunological and nutritional factors [14]. Some reports mention that there is nutritional and biological deterioration of human milk after pasteurization and thawing, even during storage. The Holder pasteurization inactivates milk lipoprotein lipase, an enzyme of major importance to the infant assisting in the hydrolysis and absorption of milk fat in the small intestine, resulting in a reduced absorption of fat in preterm infants [15]. Levels of vitamin C, folic acid, vitamin B6, and thiamin decrease with Holder pasteurization [16]. Some biologically active, immunologic, and anti-infective factors are affected. Reports exist about the reduction in IgA levels and activities, IgG, lysozyme, lactoferrin levels and activities, lymphocytes, growth factors, cytokines, lipase level, and the activity and destruction of IgM [17,18].
In addition to this, a contamination risk can be presented due to the manipulation of the milk, because pasteurization is insufficient to eliminate 100% of microorganisms, sometimes prevailing lactic bacteria or saprophytes, which over time degrade the nutritional compounds, even can causing serious illness in infants [7]. Since for human milk to be consumed by infants, it must undergo defrosting and heating, affecting practically the entire composition of this food. In addition, it is necessary to consider the waste of breast milk that is discarded by the HMB for sanitary reasons.
For this reason, at the University Center of Exact Sciences and Engineering (CUCEI) of the Univerisidad de Guadalajara and the Hospital Civil “Fray Antonio Alcalde” of Guadalajara, Jalisco, México, we have worked on the development of a spray drying process in combination with pasteurization, to preserve this basic food for the infants, where the spray drying process is an “important alternative” for the physicochemical, nutritional, biological and innocuous preservation of a powder product, easy to handle, with a long shelf life, packed in trilaminated bags, which favor the distribution of human milk to all hospitals in Mexico, improving the health and quality of life of infants and encouraging hospitals to avoid the consumption of formula milk.
Materials and Methods
The samples of human milk used in this study was obtained of Human Milk Bank from Hospital Civil de
Guadalajara Fray Antonio Alcalde, México; collected under informed consent of donor mothers, and this
study was approved by the Ethical and Research Committee of Hospital Civil de Guadalajara Fray Antonio
Alcalde, in May 2016. Human fresh milk was thawed and pasteurized at 85ºC for 5min in hot water bath
(Heidolph Laborota 4000, U.S.A.).
Samples of human milk pasteurized were homogenized at 25ºC and dried with a Spray dryer BÜCHI Mini
Spray Dryer (B-29, Switzerland). Parameters of operating were: feed rate of 1 to 2mL/min, and air inlet
temperature of 150 to 200ºC, air outlet temperature of 60 to 80ºC.
Human milk samples were performed, plaque casting on Plate Count Agar (PCA) (BD Bioxon®), potato
dextrose agar (PDA) (Difco TM®), Macconkey (Mc) (BD Bioxon®) agar and MRS agar (MRS) (BD
Bioxon®). The plates were incubated (Hinotek DHP-9052 Heating Incubator, China) for 24 to 48 hours at
37ºC (under anaerobic conditions in the case of MRS agar) and at 30ºC PDA. After this time the existence
of microbial growth was observed.
Powder samples of HM with different treatments were reconstituted with approximately 10ml of injectable
water PISA®, to restore the original humidity of them before processing, and fresh HM were centrifuged
at 10000rpm for ten minutes at 4ºC. The supernatant obtained was used to determinate the content of
immunoglobulins IgA and IgG by nephelometry according to Montagne et al., (1992) [19] with the
equipment Vital Scientific Selectra E Chemistry Analyzer, Netherlands.
Was determined by gas chromatography. Human milk lipids were extracted by according to Folch et al.,
(1957) [20], using chloroform:methanol (2:1, v/v). The solvent phase was evaporated and the extracted lipids
were stored frozen until analysed.The extracted lipid samples were derivatized using sodium methoxide 0.5N
(Supelco, Belefonte, PA). Methylation was carried out by adding 1mL of sodium methoxide to 50μL of fat
samples and allowed to rest at room temperature for 5 minutes. The reaction was stopped by adding 100μL
of water and the methyl esters formed were extracted with 2mL of hexane; then, 1μL of the sample was
injected into the GC. Separation of fatty acids was done on a HP 6890 GC fitted with a HP-INNOWax
capillary column (60m x 0.25mm x 0.25mm film thickness) and a flame ionization detector (FID); the
program temperature for the fatty acids separation was carried out according to [21].
Was performed by HPLC chromatography, using the method reported by Feary (2010) with some
modifications. 0.2g of human milk powder was rehydrated with 1600μL of buffer (24g of urea, 0.78g of tris-
HCL, 0.65g of sodium citrate and 38mg of Dithiothreitol in 42mL of water). It was left to rest for an hour.
It was then centrifuged at 13,000rpm for 30 minutes at 4ºC to remove the fat. The samples were diluted
in a 1:3 portion with a urea buffer (18g) in 45mL of a solution of 0.1% trifluoroacetic acid in acetonitrile,
centrifuging at 13,000rpm for 10 minutes at 4ºC. Lugo samples were filtered with a 0.20 micron millipore
filter for analysis by HPLC. The HPLC equipment used is the Varian brand, Protar 210 model, with Perkinelmer
Ic-95uv / visible spectrophotometer UV detector. A C18 Microsorv-MV 100-5 reverse phase column
(150 x 4.6mm, Agilent) was used at 35ºC. The mobile phase consisted of phase A (0.1% trifluoroacetic
acid in water) and phase B (0.1% trifluoroacetic acid in acetonitrile) in a gradient system at a flow rate
of 1mL/min. The injection volume was 20μL and the samples were analyzed at a wavelength of 220nm.
The identification and quantification of lactoferrin was done by comparison with the retention times of a
standard of lactoferrine (SIGMA).
3g sample was placed in a drying oven at a temperature of 60ºC until a constant weight was obtained. The
samples were weighed before and after the drying treatment, the difference in weight (the weight loss) was
calculated and the humidity was determined as a percentage of the weight of the powder according to the
formula:
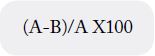
Where:
A is the weight of the initial sample
B is the weight of the dehydrated sample
The ash analysis was carried out with the dry samples human milk obtained from the moisture analysis, which
were incinerated by placing the crucible directly to the flame to eliminate the organic matter. Subsequently,
the crucibles were subjected to a muffle at 500ºC±25ºC for one hour. The mass of the crucible empty and
with the ashes, was determined in the analytical balance. The ashes were determined as a percentage of
the difference of the crucible weights without and with the dry sample (C-A) between the weights of the
crucible without and with the calcined sample (B-A) per 100:
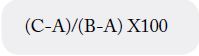
Where:
A is the value of the crucible mass
B is the value of the mass of the crucible with the sample
C is the value of the crucible mass and the calcined sample
Lactose was quantified using the phenol-sulphuric acid method [22]; pH values were determined using a
Thomas Scientific TS675 pH meter; proteins were measured by Lowry’s method. The water activity on the
samples of human milk powder was analyzed with the help of an AquaLab Pre Water Activity Meter of the
Decagon Devices brand.
Comparison of means by Tukey’s test (p<0.05) was carried out using the Minitab statistical software v.16
(Minitab Inc., State College, PA).
Results and Discussion
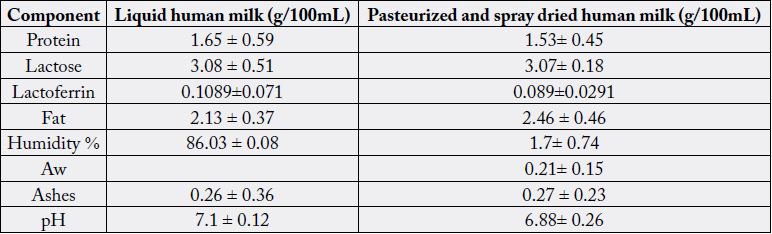
The results obtained from the total content of protein, lipids and carbohydrates in human milk (Table 1),
after the pasteurization process plus spray drying, did not show significant changes after of these processes.
The concentrations of macronutrients in human milk can be influenced by various processes, such as storage,
freezing, and thawing. Human milk, when submits to storage, freezing, and thawing processes, demonstrate
a significant decrease in fat content up to 9% reduction, most likely due to the adherence of breast milk to
the container wall, lipolysis, or lipid peroxidation. Likewise, there were statistically significant increases in
protein concentrations, maybe thawed frozen breast milk could cause the aggregation of casein micelles,
resulting in variations in the protein content [23].
Abranche et al., [24] analyzed the changes in human milk macronutrients: fat, protein, and lactose in natural human milk (raw), frozen and thawed, after administration simulation by gavage and continuous infusion. The fat content was significantly reduced (p<0.001) in both raw and thawed samples. The thawing process significantly increased the levels of lactose and milk protein, as well as showed loss of fat.
In the case of the content of lactoferrin (Table 1), the process of spray drying decreases the concentration regarding milk without treatment, but nevertheless, we managed to retain 82% of this component in human milk powder. Lactoferrin is a glycoprotein which is present in milk of mammals, that contributes to the absorption of iron in the child’s intestine and fixes it, avoiding that is used by bacteria. It has a strong bacteriostatic effect on Staphylococcus and E. coli, depriving them of iron [25].
It has been reported that concentrations of lactoferrin concentrations in colostrum and mature milk vary of 3.1-6.7mg/mL, and 1.0-3.2mg/mL, respectively [26]. In cow milk, lower ranges of lactoferrin have been found, these values vary between 1.15μg/mL to 485.63μg/mL in milk from healthy animals [27]. If we compare human milk powder with commercial milk formulas, regarding the content of lactoferrin, our result (0.8mg/mL) is really interesting, since the milk formulas do not contain lactoferrin, because they are made with cow’s milk in dust and the concentration of this component is very low, destroying probably during the process of the same.
On the other hand, we analyzed by HPLC and gas chromatography the lipid fraction in the human milk [28]. With the objective of this research was to study the effect of pasteurization, freeze-drying and spray drying on some characteristics of human milk fat, as by as the fat content, globule size and distribution were measured. Non significant differences in fat content between the different processes were found; however, there was a decrease of 23% in the fat content of spray dried milk, regarding the pasteurized milk, this due because part of the fat was trapped in the feeding hoses to the dryer. The fat mean globule size decreased considerably in all treatments, varying from 2138 to 529nm. The size distribution of fat globules increased during pasteurization and drying from 0.24 in raw milk to 0.78 in pasteurized milk. With respect to the fatty acid profile, we found that human milk samples had an elevated content of palmitic (27%), and oleic (30%) acids and significant variations were observed in the pasteurized samples for oleic and linoleic acid. Preservation processes applied to human milk caused a decrease on the fat globule diameter; the change in size increased the surface area and could improve the bioavailability of the fat components. This is the first report published on fat content and lipid profile in human milk spray dried [28].
Freeze-drying and spray-drying methods were investigated in relation to the retention of immunoglobulins (Ig) A, IgG, and IgM [29]. Spray drying produced human milk powders with 2% humidity and a good retention of IgG (>88%) and IgM (∼70%). However, only 38% of IgA remained after spray drying. For freeze drying, only the highest heating plate temperature used in this study (40ºC) brought IgA content down to 55% in powder with 1.75% residual humidity, whereas milk samples undergoing lower temperatures had higher preservation rates (75% for IgA and 80% for IgG and IgM) and higher residual moisture contents. From these results, it can be concluded that IgA is the most sensitive Ig lost during drying processing of human milk. The best method to generate human milk powders without a significant loss of Ig was thus freeze drying at 30ºC heating plate temperature, which accelerated the process compared to lower processing temperatures, but still had good overall Ig retention [29].
Another study carried out in our laboratory by Castro-Albarrán et al., (2017) [30], on the content of immunoglobulins and C3 in pasteurized human milk at different temperatures (62.5, 72 and 85ºC), showed that the total protein content presents decrease without difference meaningful While the content of immunoglobulins IgA, IgG, IgM and C3 tend to decrease with the three pasteurization processes (62.5, 72 and 85ºC) with respect to the initial content. Regarding the lyophilization process after pasteurization at 62.5, 72 and 85ºC, a decrease in the IgG and IgM content was observed, observing a lower loss in the pasteurization process at 72ºC, at the same time the IgA contents and C3 remain constant. Proportionally, the pasteurization temperature at 62.5ºC showed better efficiency in the retention of proteins and immunoglobulins, with the following behavior pattern: 62.5ºC better than 72ºC, and this in turn better than 85ºC, for the analyzed components, total proteins, IgA and IgM, with the exception of IgG, which presented the best performance at 72ºC.
Regarding microbiological studies, human milk was analyzed before and after the pasteurization and spray drying process, according to the description in materials and methods. In samples of unpasteurized liquid milk, cocci and bacilli with different biochemical and physiological characteristics were found. In the standard agar medium, an abundant growth of white, irregular colonies was observed, without definite, convex and opaque forms, all with the same macroscopic characteristics. The Gram stain test was performed observing that most of the colonies were elongated, Gram negative bacilli. No specific identification tests were performed, however, we believe that it may be lactic bacteria and some Enterobacteria, which are microorganisms of the natural flora in human milk, since in the MRS agar we observed a high bacteria count, as well as, a smaller amount of growth was observed in McKonkey agar. Rodríguez et al., (2008) [31], indicates that among the bacteria that are most frequently isolated in human milk, we can highlight several species of genera as: Staphylococcus, Streptococcus, Enterococcus, Lactococcus, Lactobacillus, Weissella and Leuconostoc.
Novak et al., (2008) [32] detected a count of aerobic mesophilic bacteria with figures higher than 104CFU /mL in 80% of their samples analyzed from unpasteurized human milk showing a high degree of contamination in the milk. In our study, an average of 632 to 850CFU/mL was found for all unpasteurized samples. Serafine et al., (2003) [33], found in the raw milk, the presence of Staphylococcus spp. Streptococcus spp., Molds and yeasts and Enterobacteriaceae. They also pasteurized the human milk and detected in 144 samples pasteurized, 3.5% of Staphylococcus aureus, 10.4% of Staphylococcus epidermidis, 1.4% of Staphylococcus lugdenensis, 2.8% of Streptococcus spp., 25.7% of molds and yeasts and 6.3% Enterobacteriaceae in nine. This results showed a high degree of contamination in raw milk and the pasteurized milk, despite the elimination of pathogens after pasteurization.
Carvalho et al., (2005) [34], analyzed overall contamination by viable strict and facultative aerobic microorganisms in samples of human milk discarded by the bank of human milk. Of the 66 samples analyzed, was 23 positive samples, among these, 19 were positive in the confirmatory test for total coliforms, which gives about 29% of total samples. They also found 18% of thermotolerant bacteria, with the presence of Salmonella, Shigela spp., and Staphylococcus aureus. This results shows the need to obtain a milk with a lower initial microbial load so that the pasteurization is efficient in the microbiological control, or implement a more efficient conservation method such as spray drying.
According to the guidelines for the operation of human milk banks, if the milk has been pasteurized by Holder pasteurization, it must be stored at -20ºC. There is no general consensus for the best storage time of breast milk based on current research. But nevertheless, it is mentioned that milk once pasteurized must be refrigerated at 4ºC in a the maximum storage period has been at 24 hours. However, some authors mention that the fresh mother’s milk may be stored at refrigerator temperature for as long as 96 hours, with minimal changes in your composition and retaining your overall integrity [6,35]. Whilst Abramovich and Friel (2011) [36] express that the acceptable storage time for pasteurized human milk in refrigeration can be up to 8 days. For this reason, it is recommended to use a frozen storage of maximum 3 months for pasteurized human milk is recommended, intended for the feeding of premature infants [37].
Our results showed that the temperature of pasteurization at 62.5 ºC/30min is not the most adequate to kill 100% of the natural flora in the human milk, since at this temperature, it reaches a reduction of 98.67% of total flora (Figure 1). While at the temperature of 72ºC/15min and 85 ºC/5min the reduction was 99.77% and 99.98% respectively, reducing the total concentration of microorganisms to 3 logarithms. The best pasteurization condition for human milk is at 85ºC/5min, since it manages to eliminate the highest amount of microorganisms and also does not decrease the immunoglobulin content.
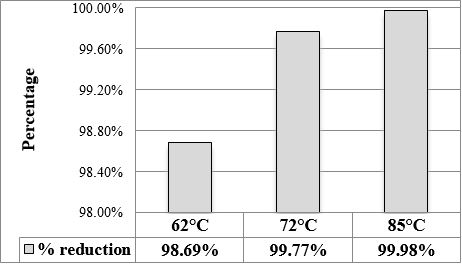
After spray drying there was no growth in any of the media used. Subsequently, it was carried out monitoring for 12 weeks at room temperature, packing 5g of human milk powder in sterile trilaminate bags and analyzing each week to observed if there was bacterial growth. After 8 weeks there was development of lactic bacteria, but not of Enterobacteria, neither mushrooms nor yeasts. It is important to mention that our work is unique in the world, since no studies similar to ours have been found.
The human milk is considered as a food product extremely susceptible to processes of conservation, thus its quality decreases with passage of storage and, as such, it has a limited shelf-life, regardless of the preservation methods used and the control of storage conditions. Is very important that the human milk preserve its protective, digestive, inductive and nutrient carrier properties during the storage. As to date only limited processing procedures have been developed for human milk conservation, being the key factors that impact the shelf life of human milk are packaging and the storage temperature [38,39].
Conclusion
This research has shown that the process of spray drying of human milk in combination with pasteurization,
can be an important alternative for the conservation of this food so vital for newborns with breastfeeding
problems. The content of protein, carbohydrates and lipids remain unchanged in human milk after the process of spray drying, as well as the content of lactoferrin and immunoglobulins, was possible to retain up to
80% in human milk powder. Human milk powder could replace milk formulas, since it has a nutritional and
safety quality. Further offering to Human Milk Banks a better alternative for the conservation and use during
the feeding of premature children who are hospitalized or for some reason their mothers cannot breastfeed.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!