Biography
Interests
Ayse Istanbullu Tosun & Filiz Yarimcan*
Department of Medical Microbiology, Istanbul Medipol University, Turkey
*Correspondence to: Dr. Filiz Yarimcan, Department of Medical Microbiology, Istanbul Medipol University, Turkey.
Copyright © 2020 Dr. Filiz Yarimcan, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
There is a need for serological tests to diagnose and manage epidemiologically COVID-19.
Several high throughput technologies developed SARS-CoV-2 antibody kits, the performance
of which has not been studied yet. We aimed to evaluate MAGLUMI SARS-CoV-2 IgM/IgG
Chemiluminescent Immunoassay (Snibe, China).
We tested sera of confirmed COVID-19 cases (n=39) and control samples of healthy subjects
(n=56). The samples were collected at 39.2 ± 15.2 (range, 14 to 56) days after the symptom onset.
We calculated test specificity as 98.2% for IgM and 100% for IgG. Test sensitivity was found as 33.4% for IgM and 71.8% for IgG. IgM was detected in samples collected earlier in the progression
of the immune response.
MAGLUMI SARS-CoV-2 showed high specificity and low sensitivity. However, with many
unknowns about the immune response of COVID-19, we could not estimate the real sensitivity of
the test.
Abbreviations
SARS-CoV-2= Severe Acute Respiratory Syndrome Coronavirus-2
COVID-19= Coronavirus Disease 2019
IgM= Immunoglobulin M
IgG= Immunoglobulin G
CLIA= Chemiluminescence Immunoassay
ABEI= N-(4-aminobutyl)-N-ethylisoluminol
PCR=Polymerase Chain Reaction
Introduction
The antibody response to SARS-CoV-2 is not entirely understood. In COVID-19 patients, antibodies can
be detected 9-10 days after the symptom onset. Seroconversion for IgM and IgG is generally simultaneous
[1]. Antibodies against SARS-CoV-2, like seasonal Coronavirus antibodies, decline with time. IgM declines
after a month and IgG after approximately two months [2]. During the 10th to 60th day after the symptom
onset, the serological tests can help diagnose and epidemiologically investigate COVID-19.
There are high throughput platforms by which anti-SARS-CoV-2 antibodies can be detected. Besides, there are also numerous rapid cassette tests. The clinical and analytical performance of those tests is not well established.
In this study, we aimed to analyze the performance of China-based Snibe Diagnostics' MAGLUMI SARS CoV-2 IgM/IgG kits. This assay is a chemiluminescence immunoassay (CLIA) that uses ABEI (a new isoluminol) molecules for labeling. It targets antibodies against both nucleocapsid and spike proteins of the virus. It requires a special autoanalyzer on which other tests, such as cytokines, cardiac, and coagulation parameters, can be studied.
Materials and Methods
We used a collection of serum samples obtained from patients documented to be COVID-19 by having
positive PCR result after a typical clinical presentation (n=39) and serum samples from randomly selected
healthy individuals (n=56). We recorded the days that passed from symptom onset to the day of blood
withdrawal using hospital and laboratory patient records for positive sera. All samples were tested for antibodies by MAGLUMI SARS-CoV-2 IgM/IgG kits on the Maglumi™ 800 analyzers (Snibe Diagnostic,
Shenzhen, China). The thresholds of positivity for these automated immunoassays are 1.0 AU/mL for IgM
and IgG.
Istanbul Medipol University Non-interventional Research Ethics Committee has approved this study (10840098-772.02-E.34485).
We tested each sample for both IgM and IgG and calculated sensitivity and specificity for each antibody type separately, assuming that both IgM and IgG are present in all positive samples. We further tested the samples with unexpected results, namely positives from the negative group and negatives from the positive group, by Elecsys anti-SARS-CoV-2 (Roche Diagnostics, USA) and Abbott anti-SARS-CoV-2 IgG (Abbott Diagnostics, USA).
We performed statistical analysis with Excel 2013 (Microsoft Office). Continuous variables (days from symptom onset) were expressed as means ± standard deviations. We compared two variables using Student's t-test and more than two variables using one way ANOVA test. A two-sided P value of less than 0.05 was considered statistically significant.
Results
Using the contingency tables for both IgM and IgG (Table 1 and Table 2), we calculated 33.33% (95%
CI 19.09-50.22) sensitivity and 98.21% (95% CI 90.45-99.95) specificity for IgM, and 71.79% (95% CI
55.13-85.0) sensitivity and 100% (95% CI 93.62-100) specificity for IgG.
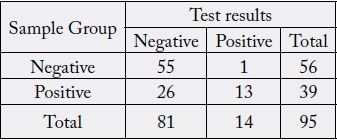
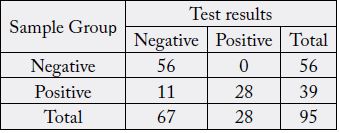
In the negative group (n=56), we had one false positive IgM result; all IgGs tested negative. In the positive group (n=39), we obtained positive results for both IgM and IgG in 13 samples, 15 samples tested positive for IgG only, and 11 samples were found negative for both IgM and IgG. There was not a sample that tested positive for IgM only (Table 3).
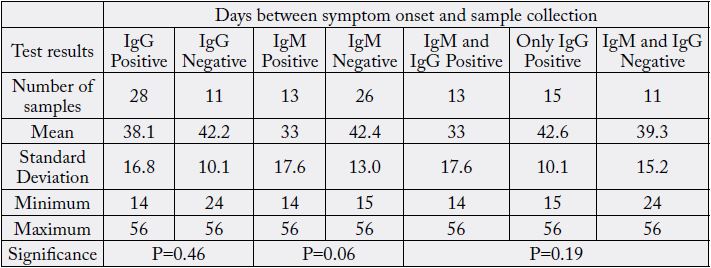
From symptom onset to sampling for all samples in the positive group, the mean duration was 39±15.8 days, from 14 to 56 days. Table 3 summarizes all comparisons done with those time intervals when the positive group is subdivided into the groups according to the MAGLUMI CLIA SARS-CoV-2 antibody kit results.
Eight samples negative for both IgM and IgG from the positive group were positive with Roche and Abbott platforms; one tested positive with Roche and negative with Abbott, two were negative by both of the additional platforms. All of the samples positive for IgG but negative for IgM in the positive group (n=15) were also positive with Roche and Abbott. The sample from the negative group positive for IgM with MAGLUMI was negative on Abbott and Roche platforms.
When we compare the duration between symptom onsets and sampling in samples with positive versus negative IgG results, we see that the positive samples were collected earlier (38.1±16.8 days) than negative samples (42.2±10.1 days). When we compare the samples that tested IgM positive with those that tested IgM negative, the IgM positives were collected earlier than the negative ones, 33±17.6 vs. 42.4±42.4 days. The samples from the positive group positive for both IgM and IgG were collected 33 days after the symptom onset. The samples from the positive group negative for both IgM and IgG (n=11) were collected approximately 39.3 days after the symptom onset. The samples positive for only IgG (n=15) were collected 42.6 days after the symptom onset. None of those differences were statistically significant.
Discussions
In this study, we evaluated the performance of MAGLUMI SARS-CoV-2 IgM//gG on a panel of 95 samples
(39 confirmed COVID-19 patients and 56 randomly selected asymptomatic patients). We calculated 33.3% sensitivity and 98.21% specificity for SARS-CoV-2 IgM and 71.8% sensitivity and 100% specificity for
SARS-CoV-2 IgG tests. The positive samples were collected approximately six weeks after the symptom
onset (39.3 days). The samples that tested IgM positive, although not statistically significant, were collected
earlier than samples having only IgG positive test results.
We found the test to be very specific, having only one false positive IgM result. However, the sensitivity was low. Eleven of the positive samples were found negative for both IgM and IgG. At this stage of the COVID-19 pandemic, the immune response against SARS-CoV-2 is not fully understood. Although collected from COVID-19 patients, those samples may not contain antibodies either because of incorrect timing of the sampling or antibodies may not develop in mild disease. To better understand whether those samples contained antibodies, we tested them also on Abbott and Roche platforms. On Abbott, only IgG type of antibodies, on Roche total antibodies are detected. Two of those samples from mild cases taken after 45 and 50 days from the symptom onset were also negative by Abbott and Roche. Another sample again from a mild case collected after 32 days from the symptom onset tested positive by Roche and negative by Abbott. The rest eight samples were positive by these additional platforms, so they contained antibodies that could not be detected by MAGLUMI IgM/IgG kit.
The claimed diagnostic sensitivity for IgM and IgG was 78.65% and 91.21%, respectively. The claimed diagnostic specificity was 97.5% for IgM and 97.33% for IgG [3]. Our results were close for specificity but much lower for sensitivity. It is because we tested a few samples when compared to those tested by the manufacturer.
In the literature, two other studies evaluated MAGLUMI SARS-CoV-2 IgM/IgG assay, calculating sensitivity and specificity. Padoan et al. studied 70 samples collected at the different stages of the disease. They reported 100% sensitivity for 25 of the samples collected >12 days from the symptom onset. For IgM, the highest sensitivity calculated was 88% [4]. Soleimani et al. evaluated MAGLUMI SARS-CoV-2 IgM/ IgG on 100 negative and 176 positive samples. They reported 100% specificity and 62.5% sensitivity for IgM, and 60.2% sensitivity for IgG. In this study, all of the positive samples were collected up to the 25th day from the symptom onset, which explains the IgG's relatively low sensitivity [5]. In our study, all of the positive samples (n=39) were collected 4-6 weeks after the symptom onset and were expected to be IgG positive. However, eleven samples were missed by the test leading to low sensitivity results.
The IgM presence could not be controlled on a different platform. We do not know what the time interval in the disease progress with detectable IgM levels is. The real sensitivity of the test for IgM could be higher. Not having an appropriately confirmed control serum is the major limitation of our study.
Valdivia et al. performed a cross-sectional seroepidemiological survey on asymptomatic healthcare workers using MAGLUMI SARS-CoV-2 IgM/IgG assay. In their study, 40 of the 1153 individuals (3.5%) tested positive for IgM and negative for IgG. In our study, this ratio was 1.7% (1 out of 59 samples). The authors retested the samples with a rapid cassette test, and 7 of those samples were found positive for IgM and negative for IgG. They also performed a PCR test on 40 healthcare workers, and 1 of them tested positive. None of the participants developed symptoms on the follow-up. In this study, the specificity of the test is also questioned [6].
MAGLUMI SARS-CoV-2 IgM/IgG assay was also used in some method comparison studies. Lippi et al. compared the SARS-CoV-2 antibody test results obtained with MAGLUMI and EUROIMMUNE. They tested a total of 131 samples, 48 of which were PCR positive. They found that MAGLUMI and EUROIMMUNE results are in good concordance, while they could not state the same for the concordance with the PCR results [7].
Montesinos et al. compared their COVID-19 case samples (n=128) and control samples (n=72) for SARSCoV- 2 antibody results by MAGLUMI, EUROIMMUNE, and three rapid cassette tests. They collected their samples generally early in the disease course. They had 32 samples collected later than the 15th day of the disease, and 28 were found positive. For MAGLUMI, they calculated 58.7% sensitivity for IgM and 53.2% for IgG. The specificity was 100%. When statistically compared, the performance of EUROIMMUNE IgA was better than MAGLUMI IgM; for IgG, there was no difference between the two methods [8].
Conclusions
In our study, we showed that the fully automated MAGLUMI SARS-CoV-2 has excellent specificity. The
sensitivity of the test, although seeming low, cannot be interpreted at this stage of the pandemic caused by
a novel virus. We do not know the antibody kinetics in patients with different severity of the disease. We do
not know the half-life of the antibodies. We do not have a gold standard method to determine the actual
performances of newly developed and urgently approved technologies. Despite so many unknowns, it is clear
that antibody testing, IgM or IgG, cannot replace PCR for the diagnosis of COVID-19 because of the late
seroconversion. Testing for antibodies can be useful for highly suspected cases with negative PCR results
and epidemiological purposes. Also, it can be useful to determine the antibody presence in the population.
Acknowledgments
None
Conflicts of Interests
The authors have no conflicts of interest to declare.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!