Biography
Interests
Abdolreza Javadi1,2, Mohammad Hashemi-Bahremani1, Shahriar Dabiri3,4, Houman Vosough2, Manzumeh - Shamsi Meymandi4, Mona akbari2 & Nemat Khansari5*
1Department of Pathology and Laboratory Medicine, Imam Hossein Hospital, Shahid Beheshti University of
Medical Sciences, Tehran, Iran
2Imam Hossein Central Medical Laboratory, Shahid Beheshti University of Medical Sciences, Tehran, Iran
4Pathology and Stem Cells Research Center, Kerman Medical School, Kerman University of Medical Sciences,
Kerman, Iran
5Department Immunology, Medical School, Medical University of Tehran, Tehran, Iran
*Correspondence to: Dr. Dr. Nemat Khansari, Department Immunology, Medical School, Medical University of Tehran, Tehran, Iran.
Copyright © 2020 Dr. Nemat Khansari, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
To compare the laboratory characteristics and viral load between mild, severe and critical patients with COVID-19. Data from 220 laboratory-confirmed patients were retrospectively analyzed. Study was conducted on COVID-19 cases who presented to Imam Hossein Teaching hospital in Tehran (Iran) from early March to late April, 2020. The recommended laboratory tests, as well as the upper respiratory viral load in COVID-19 patients with different spectrum of disease severity were compared. Lower lymphocyte absolute count, serum vitamin D3 and higher neutrophil absolute counts were seen in critical COVID 19 Patients. CRP, ESR, LDH, Neutrophil absolute count, lymphocyte absolute count and oxygen saturation were significantly abnormal in high RNA viral load of COVID 19 cases than others. High Nasopharyngeal (NP)/Oropharyngeal (OP) NP/ OP viral loads (low RNA cycle threshold) may correlate with severity of the disease and reflect tissue damage.
Our finding suggests that PCR RNA viral load LDH, quantitative CRP, ESR, lymphocyte count and neutrophil count could be commonly used to diagnosis, for assessing severity of disease, follow up of patients and possible therapeutic guiding.
Introduction
Since the COVID-19 outbreak began in Wuhan, China, late last year [1,2], it has rapidly become
a pandemic that has now infected more than 12.26 million people worldwide and death toll has been
reported to be 554,924 as of July 10,2020 in 188 countries and regions. On February 19th, Iran reported its
first confirmed cases of the infection. As of July 10th, 2020, a total of 248,379 laboratory-confirmed cases
had been documented in Iran [3]. The epidemiological data show some countries reported higher case of
fatality rate as Iran (4.8%), Italy (6.81%, 1,441 deaths/21,157 cases), Argentina (8.33%), and Iraq (10.0%)
as examples [4].
More research is needed to reveal the risk factors, such as laboratory data and biomarkers for severs and critical COVID-19 patients. It was previously emphasized that laboratory medicine plays an important role in the early detection, diagnosis and management of many diseases [5,6]. The laboratory tests are frequently applied for assessing disease severity, defining the prognosis, and guiding treatment [7].
Based on systemic reviews of 660 articles most frequent laboratory abnormalities in COVID 19 patients were, decreased albumin, high C-reactive protein, and high lactate dehydrogenase (LDH), lymphopenia, and high erythrocyte sedimentation rate [8].
A meta-analysis of 67 articles reported 81% lymphopenia in COVID-19 confirmed patients. The systemic review demonstrated elevated CRP level in all patients within first three days of the disease onset. CRP levels more than 100mg/L were seen in approximately 50% of their COVID-19 cases. Laboratory abnormalities in D dimer, Ferritin, IL1, IL6, cardiac enzymes and coagulation tests were statistically significant in the meta-analysis [9].
On the other part, some routine laboratory tests, such as ferritin, lactate dehydrogenase, and D-dimer, were marked increased in severe and critical COVID-19 19 cases. Although the study showed absolute numbers of CD4+ T cells, CD8+ T cells, and B cells were gradually decreased with increased severity of the illness [10]. Another researches revealed that C-reactive protein (CRP) and IL-6 were increased in COVID-19 patients, whereas the absolute number of lymphocytes was decreased in severe cases [2,9].
The current studies focus on direct correlation between nasopharyngeal SARS-CoV-2 viral load and disease severity. They emphasized that high nasopharyngeal RNA viral load is more common in severe and critical Covid19 patients [11,12].
The aim of our study is to investigate the most frequent laboratory abnormalities in definite COVID-19 patients and possible correlation with NP/OP viral load and laboratory abnormalities in mild, severe and critical COVID-19 patients.
Materials and Methods
A retrospective study of 220 confirmed COVID-19 cases, in accordance with WHO guidelines for the
diagnosis of SARS-CoV-2 [13], and admitted to the Imam Hossein teaching hospital between March and
April 2020.
Routine confirmation of cases of COVID-19 is based on detection of unique sequences of virus RNA with real-time reverse transcription polymerase chain reaction (rRT-PCR) and confirmation by sequencing as needed [13].
The imported patients with COVID-19 in this research were those who had been referred or originated from city of Tehran, and were Molecular confirmed from March to April 2020.
The COVID-19 patients with different severity of illness were classified into three groups:
The clinical laboratory tests as well as NP/OP RNA viral load, were compared among mild, severe and critical and also between non-critical (Mild and Severe) and critical (ICU admitted) according to the clinical presentations [14].
Demographic data and laboratory results of COVID-19 cases were collected from Imam Hossein Hospital
LIS and HIS affiliated Beheshti Medical University. The laboratory results were included a median of seven
days after Hospital admission date.
A nasopharyngeal (NP) swab and/or an oropharyngeal (OP) swab are recommended for screening or
diagnosis of early infection [15]. These NP and OP specimens were obtained from patients at admission
time and were transported via viral-transport medium.
A total of 10μL of RNA was used for molecular analysis. A magcore® automatic extractor was used for nucleic acid extraction from 400uL throat swab eluate per sample. Reverse transcriptase-polymerase chain reaction (RT-PCR) assay of COVID-19 was confirmed according to the cycle threshold values for E and RdRP genes. The assay was performed by the core hospital Laboratory using SLAN thermocycler machine and commercial kit based on protocol of The Charité Institute of Virology in Berlin, Germany [16,17].
Real-time RT-PCR was performed using the following conditions: 50°C for 30 minutes (RT step), denaturation 95°C for 10 minutes, 40 cycles of amplification at 95°C for 15 seconds, and 60°C for 1 minute. The assay Sensitivity was 5.2 copies per reaction. Ct values were inversely related to viral RNA copy numbers with a Ct value <36 being considered positive (Kogenebiotec Co, PowerChek TM 2019-nCoV).
Laboratory analytes including CBC, Blood gas, QCRP, CPK, Troponin I, LDH, AST, BUN, Creatinine, PT, PTT D-dimer and Vitamin D3 are selected for analysis considering to common laboratory abnormalities in COVID-19 patients [5] and recommendations for COVID-19 laboratory test panel [18].
In critical patient another prognostic test consists of procalcitonin and IL6 are included for statistical analysis.
Blood EDTA samples were collected from patients. Hematology parameters including White blood cell count (WBC), Lymphocyte absolute count (LAC), and Neutrophil count (NEU) were performed with Siemens ADVIA2120® applying peroxidase flow cytometry method and automated ESR readers.
Coagulation tests were carried out by automated SYSMEX CA500® analyzer using Siemens regents. Blood biochemistry tests were assessed using HITACHI 919 and Biolis 24 in automated random-access analyzers. All samples were labeled using barcode tracking system and the then analyzed in duplicated manner at the hospital core laboratory. The levels of IL6, procalcitonin, Vitamin D3, Troponin I and d-dimer in serum were measured. The tests were carried out by means of quantum luminescence automation (Siemens ADVIA Centaur® immunoanalyzer).
Commercial Siemens, SERO® QC materials for internal QC and RANDOX® external quality control programs (RIQAS) were applied for laboratory quality control.
Data were expressed as frequency and mean± standard error and compared using the T test or one-way
ANOVA, followed by LSD post-hoc. Categorical values were analyzed using Fisher’s exact test or χ 2 test.
To asses correlation, Pearson spearmen correlation test was used. All analyses were performed using SPSS
23.0 software (Chicago, IL, USA). A two-sided p < 0.05 was considered statistically significant.
Results
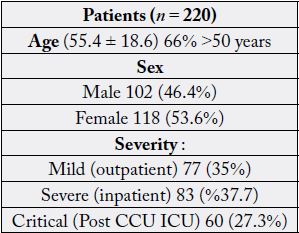
The number of 220 patient infected by COVID-19 were entered in the study. Males were 46.4% and females
were 54.6% of the samples. Based on ward admission the out patients were 77(35%), in patients were 83
(%37.7) and critical patients were 60 (27.3%). Mean age of participant was 55.4±18.6 and 66% were over
50 years old. (Table 1). Comparison of variables between over 50 with under 50 years old showed that CRP,
Urea and creatinine were increased significantly in those over 50 years old (Table 2). There was a significant
difference based on age (>50 and <50) in hospital admission since most of the critical patients were over 50
years old and most of out-patients were under 50 years old).
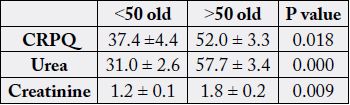
The out ranged abnormality in CBC was Neutrophil percent and ESR of all patients. While in biochemistry blood analysis, QCRP was massively increased (×10) in critical patients compared to normal range (Table 3). Also, LDH increased based on severity of the disease to 827 ± 88 U/L in critical patients likewise CPK that reached to 346±76 in critical patients. Similarly, in less dimension, the Urea, AST were increased compared to normal range. The Oxygen saturation was decreased in all severity categories (Table 3).
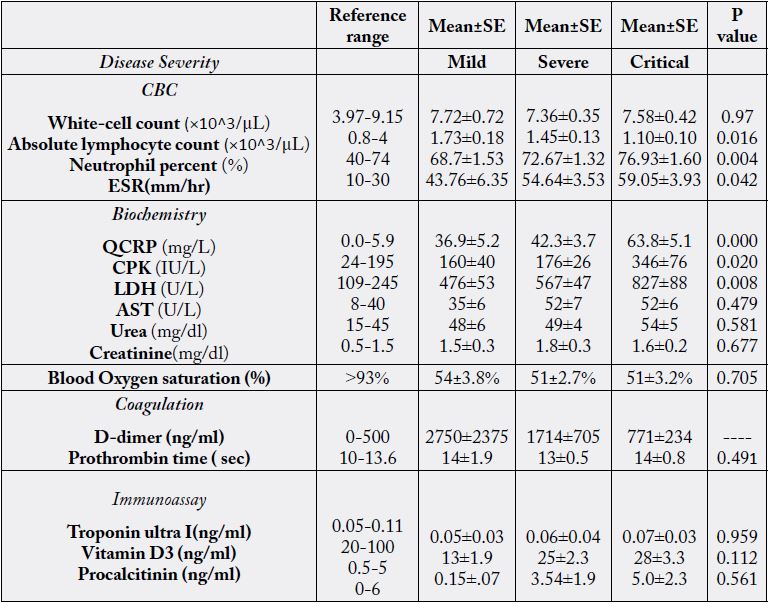
Comparison of CBC parameters among three COVID-19 severity groups (Table 3) showed that Neutrophil percentage in critical illness was increased compared to mild and severe groups (Pv= 0.016004). While in critical patient Lymphocyte counts was decreased and ESR was increased compared to mild patient category (Pv= 0.004 016 and Pv= 0.042 respectively).
Among Biochemistry analytes QCRP, CPK and LDH of critical patients were increased compared to noncritical, mild and severe groups (Pv= 0.000, Pv= 0.020 and 0.008 respectively). No difference was observed for all other variables (Table 3).
There was no age and or gender difference in cycle threshold values (RNA viral loads). Interestingly, lymphocyte count decreased (Pearson correlation= -0.218, P=0.004) and also Oxygen blood saturation decreased (Pearson correlation= -0.278, P=0.000) in correlation with nasopharyngeal viral load in COVID-19 patients.
Neutrophil percentage was significantly increased (Pearson correlation= 0.76, P=0.02), as well as LDH (Pearson correlation= 0.341, P=0.02) and Vit D3 (Pearson correlation= -0.235, P=0.046) that negatively correlated with all patient with high viral loads.
Based on RNA CT value grade, ANOVA showed significant differences between following four viral load (CT value) ranges (Figure 1); The mean of Lymphocyte absolute count of Low viral load group (Ct v >30) was increased compared to mid and high viral load groups (Pv= 0.002 and Pv=0.001 respectively).
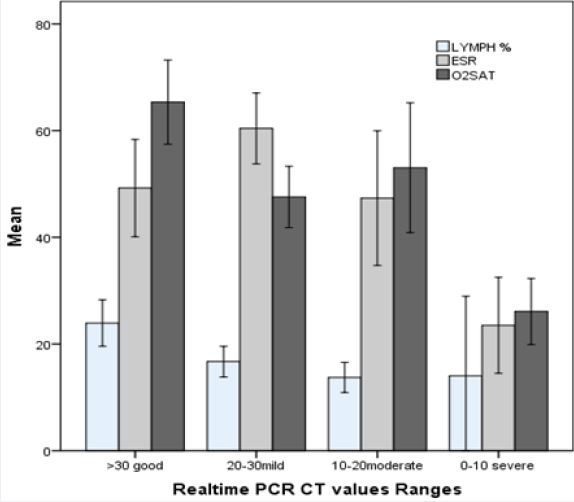
*significantly different compared to all other groups
#significantly different compared to mild group
Among acute phase factors, ESR of mild viral load group was higher compared to high viral load samples (Pv=0.046). Lactate dehydrogenase of high viral load group was increased compared to low viral load groups (Pv=0.02). Finally, Oxygen saturation was higher in low viral load group compared to all other groups (Pv<0.05) (fig 1).
Based on severity of the illness if we consider those admitted in ICU and post CCU as critical and all others as non-critical group the main finding is: The mean age of critical group was increased (59.3 ± 1.9year) compared to non-critical (54.0 ± 1.6year) (Pv=0.034). In addition, the mean of lymphocyte absolute count was decreased and neutrophil percentage was decreased and increased in Critical patients compared to Non-critical groups (Pv= 0.011 and Pv= 0.004 respectively). Same results were found for CPK and LDH that were increased in critical illness category (Pv= 0.032 and Pv= 0.007 respectively). Also, viral RNA CT values decreased (pv= 0.049) in critical group (CTv=24.9 ± 0.9) compared to non-critical groups (CTv=26.8 ± 0.5). (Median 25.5 and 27.7).
Discussion
The diagnosis of patients with COVID-19 requires laboratory molecular confirmation tests for SARSCoV-
2 RNA [13]. Moreover some laboratory tests are applied for assessment of COVID 19 severity,
complications and management [5].
Based on meta-analysis, most abnormal laboratory tests in first week of COVID-19 were lymphopenia increased CRP, LDH that consistent with our findings [8,9,10].
Similarly, the previous studies from SARS outbreak in 2003 demonstrated that the viral disease SARS may be associated with lymphopenia, leukopenia, and thrombocytopenia, elevated levels of LDH, ALT, AST, and CK [20,21].
Most important immune cells including T lymphocytes CD 8 and NK cells provide first line defense against viral organisms [22,23]. Moreover, T helper and T suppressor cells were decreased in clinically severe and critical COVID-19 patients. One of the probable pathogenesis can be direct T cells damages by the virus, leading to severe and critical illness [10,24,25].
Many published articles reported significant case fatality of elderly patient [26]. Our results showed that mean age of Critical COVID-19 patient was higher than Non-critical cases. Low lymphocytes reserve and dysfunction of lymphocytes consist CD8+ T cells in elderly people can be related to low immune system deference causes the high mortality among them [27].
In critical patients’ inflammatory mediators’ responses and cytokine storm induct Lymphocytes apoptosis and anergy [10]. It can be the reason of marked lymphopenia in our ICU admitted cases.
Interestingly our study demonstrated that upper respiratory RNA viral load was higher in the critical group than it was in the non-critical patients who showed the considerable lymphopenia. This study, consistent with some other researches, suggests the low absolute lymphocyte count by high viral load may be related to pathogenesis and disease severity [28].
Some researchers suggest that the pathogenesis of extremely severe SARS-CoV-2 infection is involved with the exacerbation of inflammatory responses and cytokines [10].
In addition, virus spikes attach to ACEII receptors on respiratory epithelium lead to cell infection, this induce cytokine response that may cause lymphopenia and neutrophilia during illness [29,30].
Although we identified that the SARS-CoV-2 RNA load was positively correlated with ESR, CRP, lactate dehydrogenase levels and reduced oxygen saturation in Critical patients, which may indicate nasopharyngeal SARSCoV-2 viral load can be related pathogenesis of the multi-organs damage.
According to previous researches, marked increased LDH, cardiac biomarkers (CPK) and acute phase reactants (CRP, ESR) might be associated with disease coagulopathy, myocardial injuries and cytokine exacerbation [31].
A histopathological study demonstrated viral particles with in capillary and endothelial cells by SEM method. Immunohistochemical study displayed induction of endothelial apoptosis and pyro ptosis, that might have a major role in endothelial cell injury and cardiovascular complications in patients with COVID-19. These pathological findings suggest that multi-organs endotheliitis may be a direct consequence of viral involvement [32].
Nasopharyngeal SARS-CoV-2 RNA load may be useful to monitor the COVID-19 disease, our findings can provide laboratory evidences contributing to the clinical diagnosis and management of COVID-19. More comprehensive laboratory studies are still needed for disease severity, prognostic factors and respiratory viral load for clinical applications.
Conclusions
Lower lymphocyte absolute count, serum vitamin D3 and higher neutrophil absolute counts are seen in
critical COVID-19 Patients. CRP, ESR, LDH, Neutrophil absolute count, lymphocyte absolute count and
oxygen saturation were significantly abnormal in high RNA viral load COVID-19 cases than others. High
Nasopharyngeal (NP)/Oropharyngeal (OP) NP/OP viral loads (low RNA cycle threshold) may correlate
with severity of the disease and reflect tissue damage.
Our finding suggests that PCR RNA viral load, LDH, quantitative CRP, ESR, lymphocyte count and neutrophil count could be commonly used for diagnosis, assessing severity of the disease, follow up of patients and possible therapeutic guiding.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!