Biography
Interests
Shimon Shatzmiller*, Inbal Lapidot, Galina Zats, Rami Krieger & Ludmila Buzhansky
Department of Chemical Sciences, Ariel University, Israel
*Correspondence to: Dr. Shimon Shatzmiller, Department of Chemical Sciences, Ariel University, Israel.
Copyright © 2020 Dr. Shimon Shatzmiller, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Four years ago the world was confronted by the Ebola outbreak in West Africa. Panic broke out all over the world. Governments have intervened to contain the pollution. Until the last patient tested negative for the disease, the outbreak claimed thousands of lives and caused billions of dollars in financial losses. President Obama Warned the U.S. to prepare for a pandemic back in 2014 [1], Bill Gates [2,3], and many others warned the world of the impending world pandemic caused by viruses. They demanded that the scientific, industrial and economic should prepare, some work has done but in low gear, too slowly as we experience today.
The Epidemic Is Here. Here's How We Can Make Sure That We're Ready
Efforts are already underway, not specifically for this outbreak, but to think of similar universal approaches
in the same way we talk about flu vaccines.” NIAID Embryo Allen
The Coronavirus has epidemic potential, and Coronavirus is likely to continue to emerge. Since we’ve seen three outbreaks of the coronavirus in the last two decades, I think it’s also essential to think in the longerterm about how we deal with this Coronavirus that will continue to come out of animal reserves. Efforts are already being made, not approaches in the same way we talk about flu vaccines [4,5].
Vaccine platforms or manufacturing technologies being tested to improve vaccine efficacy are heterogeneous between different species and/or either tailored for epidemic or pandemic (for example influenza). Here, we discuss current vaccines to protect humans and animals against influenza, coronaviruses, highlighting challenges faced to effective and uniform novel vaccination strategies and approaches.
Despite advances in training-therapeutic approaches, influenza continues to cause Severe illness, especially among uncompromising people, young children, and Elderly adults. Vaccination is the most effective way to reduce morbidity and Mortality caused by influenza viruses. Frequent genetic alteration and influenza among influenza virus strains with the gap created between virus strains and vaccines Limits the effectiveness of conventional flu vaccines. One approach Overcoming this limitation is developing a universal flu vaccine that can protect all subtypes of influenza viruses. Moreover, the development of new technologies can significantly facilitate new or improved universal influenza vaccines, including virus-like particles, cellstimulating peptides, and recombinant proteins, Synthetic viruses, widespread antibodies, and nucleic acidbased vaccines. This review has been discussed recently with the National Institute of Allergy and Infectious Diseases (NIAID) [6].
Nearly a year ago to date, High-Tech bio companies has raised the initial millions to take the universal vaccine candidate through the qualifiers.
A R&D organization at Lyon, a French-based Osivax, dropped out of Imaxio in 2017 to use what it calls the platform the oligoDOM (after the Australian sneak) technology.
Osivax, like other universal vaccine developers, strives to equip the immune system to recognize and target a conserved portion of the inanimate virus. In place of the antigens that are beginning to develop rapidly in the center of existing preventive shots.
1) A virus-like-particle (VLP) approach making the antigen much more visible for the immune system
2) A positively charged tail facilitating entry in dendritic cells to stimulate CD8 T-cell responses.
To do this, Osivax linked a recombinant form of conserved nucleoprotein with what uses virus-like particles and positively charged tails. By Targeting nucleoprotein, a highly conserved antigen with a low mutation rate found in all mu variants, oligoDOM has the potential to provide. Prolonged immunity regardless of mutation of virus. The company initially focused on, u, but has now added a new target: coronary virus, both the virus family and, specifically, SARS-CoV-2, which is currently causing the COVID-19 epidemic. Osivax takes the same approach as with towards a universal vaccine against virus protection Against SARSCoV- 2, as well as future coronavirus virus strains.
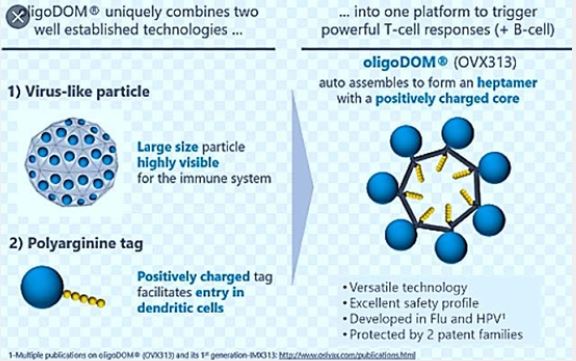
oligoDOM® (OVX313) is derived from our first generation technology (IMX313). The technology has been enhanced with the addition of a positively charged polyarginine tail to facilitate entry into dendritic cells and cause much higher CD8 T responses. OligoDOM® Technology Platform (OVX313). It is a versatile technology that can be used with a complete antigen or a long peptide Activates immune responses against cells and antibodies, with unprecedented cytotoxic T cell activation (CD8) Different immunization technologies can be used (recombinant proteins, RNA and DNA vaccines, viral vectors ...) as they can be encoded as DNA or RNA sequences Relys on more than 12 years of R&D and is protected by two patent families Currently in clinical development in two indications (malaria (first generation) and influenza)
When associated with an antigen, oligoDOM® produces a large and positively charged recombinant protein that activates the 3 immune arms with unprecedented CD8 T cell responses.
The mRNA technology may provide an effective vaccine focusing on SARS-CoV-2 is the causing agent of the current COVID-19 pandemic. Multiple recombination events may have led to SARS-CoV-2 to cause a mysterious illness in humans [11]. SARS-CoV-2 rewires host proteins to promote infection [12]. Since SARS-CoV-2 disguises its own genetic material to facilitate infection [13]. The application of the mRNA-based (“self-amplifying” mRNA molecules [14]) vaccination [15] that causes the human cell to create pathogen-eradicating antibodies is applied. The vaccines are urgently needed to control COVID-19 The ongoing and widespread epidemic of MERS / SARS caused by corona virus infections. The domain that requires CoV Spike (RBD) receptors is attractive The target of the vaccine is undermined by a limited vaccine. We describe a dimeric form of MERS-CoV RBD Overcoming this limitation. The RBD dimer significantly increased antibody neutral headings (NAb) Compared to a conventional monomeric form and mice are protected against MERS-CoV infection. Crystal structure Show motifs associated with dual receptors of RBD-dimer, the primary target for NAbs. Understood Further design yielded a stable version of RBD-dimer as a single chain with a repeating tandem (RBD-sc-dimer) Which maintained the strength of the vaccine. We have incorporated this strategy to design vaccines against COVID-19 and SARS, achieves 10- to 100-fold improvement of NAb headers. RBD-sc-dimers in pilot scale production yielded High yields, supporting their scalability for further clinical development. The framework of immunogen design Can be applied universally to other beta-CoV vaccines to deal with emerging threats. Introduction In recent years we have been dealing with unprecedented threats of corona virus (CoV) infections. During 2002- 2004, acute respiratory distress CoV syndrome (SARS-CoV) was first reported in China And have led to more than 8,000 infections worldwide with ~ 800 related deaths (https://www.who.int/). In 2012, Middle Eastern Respiratory Syndrome CoV (MERS-CoV) has emerged Saudi Arabia and spread rapidly to 27 countries, with The mortality rate is even higher at 34.4%. Infections of humans by MERS-CoV recently reported. Currently, the virus disease in 2019 (COVID-19) is caused by SARS-CoV-2 infection is classified as a spreading epidemic.
Highlighting the urgent need for vaccine development. In CoVs, the envelope-embedded spike (S) proteins are responsible for recognition of host cellular receptors to initiate virus entry [16] The receptorbinding domain (RBD) of S protein is required for the receptor docking. SARS- CoV and SARS-CoV-2 apply the same functional host cellular receptor [17], human angiotensin converting enzyme 2 (hACE2), however, MERS-CoV uses human CD26 (also known as human dipeptidyl peptidase 4, hDPP4) [18]. It was revealed the structural bases for the receptor recognition by these CoVs. So far, most of potent neutralizing monoclonal antibodies are against CoV RBD. Therefore, RBD is an attractive vaccine target because it can focus the immune response on the interference of receptor. To date, some RBD-based vaccines reported in development against MERS and SARS [19]. However, vaccines RBD-based subunits may face some important challenges, mainly due to their relatively low vaccination, which must be combined with appropriate preparations or adapted to appropriate protein sequences, fracture lengths and vaccination times [20]. Strategies to promote immunogenicity of RBD-based vaccine include increasing the antigen size, multimerization or intensive antigen display in particles, however, these strategies inevitably introduced exogenous sequences which complicated their potentials for clinical usage.
The Design of a Universal Platform for Coronaviruses Vaccine
The universal design of beta-CoV immunogens that overcomes the immunogenicity limitation of RBD
based vaccine. CoV RBD dimers have been observed before [21,22] but their immunogenicity has not been
tested. We found a disulfide-linked dimeric form [23] of MERS-CoV RBD
Amplified the antibody response, and the antibody was neutralized (NAb), titer compared to the conventional monomal form. In the mouse model it provided protection against MERS-CoV infection and relief of lung injury. The crystal structure revealed the fully exposed RBD-dimer motifs in the Double Binding (RBM) motifs, the main site recognized by NAbs. To increase dimmer stability, the immunogen is further engineered as a version of a single-chain tandem dimmer (sc-dimer) by structured design without inserting any exogenous sequence.
Can be used in any vaccine development methodology/ platform?
To date, the pharmacological response to infectious diseases is emerging and bio-terrorism has been Characterized by a “one bug, one drug” approach, in which specific medical antidotes - Effective vaccines and treatments - developed, manufactured and deployed. However, in recent years, platform technologies have been developed that can enable this Multiple vaccines produced more quickly than a single system. The John Hopkins Center for Health Security conducted this project to clarify the promise and challenges of vaccine platform conceptual understanding of various vaccine platform technologies. With special attention to how they may accelerate the development of vaccines into a global catastrophe Biological risks (GCBRs) and outbreaks of infectious diseases arise. This report outlines, the scientific and policy issues related to platforms and how they are understood in government, Academia and industry and it provides recommendations aimed at helping realize the potential The benefits of vaccine platform technologies [25].
Conclusions
As epidemic readiness matures, the integration of emerging vaccine platform technologies The development
of emerging infectious diseases has the potential to develop a medical antidote It has a significant impact
on human resilience both at the GCBR level and other epidemic scales Threats. As vaccination platform
technologies go through continuous developmental stages. It is important to track their progress, measure
their speed of development and catalog theirs Successes and failures. The use of these technologies, although
not completely replacing traditional vaccines, contains Promise to provide fast vaccination solutions
efficiently and cost-effectively. It is important to emphasize that platform immunization technologies are
not a panacea for the threat Epidemics and infectious diseases are emerging. While these technologies
have important capabilities In order to shorten development times, it is more likely to be in months rather
than years. Vaccine platforms, despite their unique features, will be placed in the context of clinical safety
Experiments on efficiency and compliance of regulatory agencies. This report is an expert assessment of
vaccine platform technologies. We found out that there is Great promise in these technologies, regardless
of regulatory ease that may or may not possible. We hope this report will provide an important context for
evaluation, discussion and follow-up These technologies in the campaign systematically remove the danger
of infectious diseases and Increase human resilience to these threats ranging from regional outbreaks to
global catastrophic biologic risks (GCBRs) levels.
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!