Biography
Interests
Shatzmiller Shimon* & Rami Krieger
Department of Biological Chemistry, Ariel University, Ariel, Israel
*Correspondence to: Dr. Shatzmiller Shimon, Department of Biological Chemistry, Ariel University, Ariel, Israel.
Copyright © 2019 Dr. Shatzmiller Shimon, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
In the USA, 42 percent of agricultural emissions come from animal agriculture. Two-thirds of those gases are directly emitted by ruminants: animals like cows, buffalo and sheep that use bacteria in their stomachs to ferment food. That allows them to eat foods, like grasses, that humans can’t.
The rumen microbiota, i.e., the community of microorganisms that inhabits the rumen, is characterized by its high population density, extensive diversity (encompassing bacteria, archaea, protozoa and fungi) and complexity of interactions.

Animals play a significant role in carbon cycling through biomass consumption and emissions of methane [1,2]. Recent studies show that the existing inventory of bottom-up methane emissions of animals in the United States, Such as those made using Intergovernmental Panel on Climate Change (IPCC) emission factors from 2006, is too low. This could be due to outdated information used to develop these emission factors. In this study we update information on cattle and pigs by region, based on recent changes in live body mass, feed quality and quantity, dairy productivity, and animal and manure management. We then use this updated information to calculate methane emissions from new animals Cause enteric fermentation in cattle and management of manure in cattle.
Although the qualitative observations on the presence of bacteria and protozoa and volatile fatty acids, was done in the 19th century, it was not until the early 1940s, when workers in Cambridge (England) did quantitative experiments showing the nature of the acids created and their absorption and their relationship with the energy needs, Immigration studies to expand. Since then, thousands of articles have been published on the biochemical microbiology of the novel, and on nutrition and metabolism. Crops of agricultural importance studied are mainly cattle, deer and goats, and for this most attention has been paid to the first two animals. Although the camels differ from the real anatomical and phylogenetically pups, their reliance on microorganisms for the degradation of plant fibers is similar to that of faithful in the rainforests living animals (cow, sheep, goat, deer for example). And are therefore generally considered "functional".
Overall, diet, rather than geographic location or even species of roast leaves, was found to be the primary cause of the types and numbers of dominant rumen bacteria and the functions of the adult animal, and similar observations were made on animals fed on similar diets in all parts of the world. (One notable exception is the distribution of bacteria that affect the toxin of plant extracts and its degradation products). Most of the observations reported in this book will apply to general tumors and to many microbial food digestion types of internal systems.
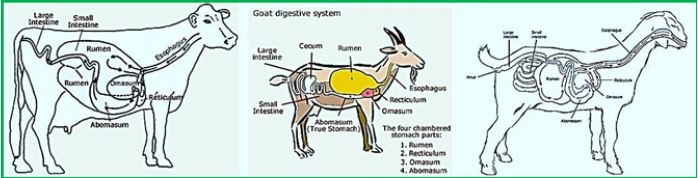
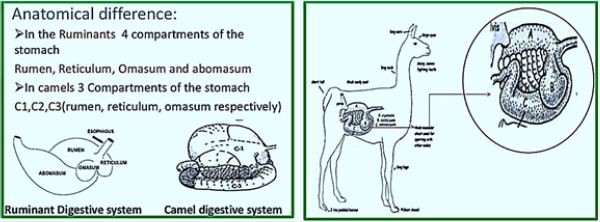
The animal digestive system supports a variety, a community of bacteria, protozoa, fungi and anecdotes [3,4]. These symbiotic organisms contribute to the nutrition of the host animal by converting non-digestible foods into absorbent compounds easily, and they thought to contribute to host physiology and health [5]. It is the symbiosis that is especially crucial for the aquatic animals, which are unable to endogenously synthesize the hydraulic enhances required for the inability of plant lignocellulosic material which forms a significant component of their diet. During fetal development, the host and the digestive system undergoes anatomy and physiology changes that allow this to enable the symbionts. These include the formation of an enlarged pre-gastric (a novel of foregut fermenters) or post-gastric (cattle and / or colon of indigestion fermentation) fermentation cell [3]. The increase in capacity Keeps the material ingested, along with the near-neutral pH Contributes to the settlement of these individual bacterial organs. Due to pH, relative to the rapid transition of digestion through the midgut or small intestine, These organs tend to be less populated by bacteria [6]. The stomach of the ruminant animal consists of four differentiated Cells, that is, rumen, reticulum, omasum and abomasum.
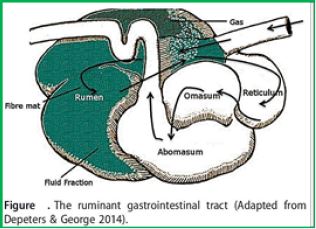
The anaerobic novel, accounts for up to 55% of an adult's volume Stomach of the throat [7] is the leading site of the microbial Fermentation of lignocellulosic material of plants. Function Of the downstream cell, omasum, is to improve the effectiveness of long-time fermentation process [8]. The "lignocellulose plant" is degraded in rumen into monomers and oligomers which later on convertible Volatile Fatty Acids chains (VFAs), a process related to the production of acetate, propionate And butyrate, along with carbon dioxide, hydrogen and Methane (CH4)[3]. VFAs serve as a primary source of energy for the host that is easily absorbed into the blood, which passes them to the liver there gluconeogenesis occurs [9,10]. The rumen bacteria also facilitate the absorption of minerals and water, provide vitamin B and contribute to the recycling of urea [3]. There they entrance, along with the digest a rumen, into the abomasum Exposing them to HCl and to the operation of the various host Digestive enzymes, thus serving as a source of proteins and amino acids for the host.
Rumen Bacteria
Bacteria are the most abundant bacteria in animal frigates, with 1010-1011 cells / ml and more than 200 species [11,12].
The composition of bacteria found in the novel is dictated by several factors, including preference for specific substrates (i.e. the diet), energy requirements [13,14,15] in which bacterial populations were studied using a culture-based approach, it was determined that a bacterial composition is mainly of Gram-positive types when animals feed high-calorie diets , with more Gram-positive bacteria, such as lactobacillus, exist in animals that feed moth diets with pH levels dropping after consumption of easily digested carbohydrates [16].
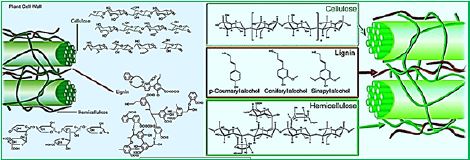
The use of molecular techniques has become critical to the analysis of rumen microbiology. These techniques can be used to determine the composition of the population present (using, for example, 16R RNA-conserved gene to determine bacterial composition) It is estimated that only 20% of rumen microbiota can be cultured using standard techniques. [17] Since the sequence of the 16S rRNA gene was first used to investigate novel microbial ecology. Extensive coverage of low abundant species has enabled the analysis of rare K. microbial auras. They also showed that the Prototella sequence, Butyrivibrio and Ruminococcus were the dominant bacteria in the rumen, and that the communal structure was affected by changes in the host's diet [18]. In particular, it has been shown that dietary complexity favors increased microbial diversity. Due to the high diets of the leaves, especially those that receive grass-based diets, cellulose digesters are an essential part of the animal's supply with essential nutrients. These bacteria reduce cellulose and mitochondria, the main constituents of plant fibers [19]. The ability to degrade cellulose depends mostly on the maturity, yield maturity, and accessibility of cellular bacterial communities [20]. The composite fiber matrix consists of β-1, 4 glucose- linked glucose residues β-1, 4 xylose is linked for hemicellulose, requiring coordination of several hydrolytic enzymes to break it [21,22].
Methane is produced at the forefront of all animal owners by current microorganisms. Dietary manipulation is considered the most effective and convenient way to reduce Methane emissions (and as a loss of energy in the animal) and increase nitrogen utilization efficiency. This review examines the effect of diet on breast function in what describes what land The microbial of the novel is known. Our understanding of this area has increased significantly In recent years due to the application of "omix" technologies to determine microbial composition And functionality patterns of rumen. This information can be combined with data on nutrition, The physiology of novel, nitrogen secretion and / or methane emissions to provide a comprehensive Insights on the relationship between microbial activity of novel and nitrogen utilization efficiency And methane emissions, with an ultimate view of developing new and improved interventions Strategies.
Signs of “Kashrut” of Beasts and Animals
The gut microbiome of animals depends on their environmental situation, namely their diet. The Tibetan [23] wild antelope has a more diverse microbiome than a Colorado domesticated cattle [24]. Moreover, captivity conditions reduce the diversity of microbes even further, compared to the microbiome of the same animals in the free pasture. A bacterial mass that demonstrated more considerable changes in the relative abundance between the captive states and the wild states included members of pyramids and bacteria. In general, the patterns presented will inform a range of disciplines from veterinary practice to captive breeding efforts. Moreover, the bacterial doses that persist in captivity provide a unique insight into symbiotic relations with the host.
Anaerobic habitats exist continuously Throughout the history of the earth, digestion ways to be a contemporary micronic [25]. In fact, all animals, including humans, Adapted to life in a microbial world. Microbial populations were described in chicken eaters, dog eaters And predators in all zoological classes including Insects, fish, reptiles, birds, rodents, bulldozers, pigs, horses, elephants, rodents, sheep, goats, cattle, camels, antelopes, monkeys and humans, and even dinosaurs. The complexity of pet relationships varies greatly, starting with competition to collaborate. The pet system has Evolved as an adaptation that allows the animal to live Food consumption and consumption is limited by other animals. This allows digestion of digested food ingested, followed by absorption and metabolism of digestion Products, while feeding and other activities continue. Since microorganisms multiply rapidly under favorable conditions in the gut They can be serious competitors for animal food. It's a microbial challenge Changed the course of evolution in animals, resulting in a different relationship between animals [26,27]. The evolutionary strategy in the first case was to compete with Bacteria in the area and in the two cooperate with them.
The Torah describes the animals (and beasts) that are pure in the verses, "This is the animal that you shall eat from all the animals that are on the ground, and every horseman who is cut off with horseshoe horseradish shall eat." (Leviticus 11: 2-3)
(Deuteronomy 6: 6) In the Book of Deuteronomy, these descriptions were changed with several differences in which the Sages and the commentators dealt. According to Maimonides, the characteristics that the Torah described are not the cause of the permit or the prohibition, but rather the signs that allow us to identify the group of pure species. "Maimonides says:" ... and know that these signs are meant to mean raising a sheep and horseshoe, (The Guide of the Perplexed Part III) The three characteristics that the Torah describes clearly define a distinct group of mammals that it allows eating - a sub-series of migratory leaves. Which I shall present as a third way of describing the status of the signs of purity (sign or reason) See also in Rabbi Avraham Stav's article " Right purity."
The first sign of a "horseshoe" means a dispute between the commentators. The Ibn Ezra (Vayikra 11: 3) thinks that "Pharisee Horseshoe" is the owner of a horseshoe: "Mervat - the adjective as well as radiating from Paris and the taste is all described as having a horseshoe." This is how the "Rashbam" interprets: "A hoof - one claw-like a shoe, not a fingernail in every finger in a rabbit and a rabbit." The "Rashbam" interpreted one hoof of a shoe as a kind of shoe and not a fingernail in each finger like a rabbit and a rabbit, and a cleft was cut, separated by two horseshoes, and not one whole horse and donkey. And according to this the solution is as follows: Every animal in its birth is a horseshoe that has a clove in its foot like a shoe that covers the leg, and also a cut that looks like two pure hooves to eat, that the foot in the clove is like a shoe, And all the language that is spread out in Shin or Banach. All of this is because the Shinn and the Sermon are one thing for them, as is the non- reward for the famine of your bread that will spread out its table before the hungry poor. The children who ask for bread do not have bread. "Regarding the connection between the" fries "and the horseshoe, we learn from the words of the Gemara (Chulin 44b):" And Rabbi Yochanan said: Is Jesus holy? We are dealing with the matter of what we are dealing with - which is laid out on the ground. "Rashi interprets:" Like the one on the ground, we shall shake the pillar and stick its hooves to stand.

The Torah lists three species of animals and seven species of animals that are permitted to eat, since they chew and slice a horseshit. On the other hand, the Torah enumerates four species of animals that have only one of the two signs: a rabbit, a rabbit and a camel, who raise a goat but do not slice horseshit. And a pig, who fries a horseshoe that is torn but does not chew.
Are There Any Particular Features to the "Kosher" (Ruminant) Animal's Microbiome?
Animals must convert these macromolecules into the simple molecules needed to maintain cellular functions, such as assembling new molecules, cells, and tissues. The conversion of food consumed on the necessary nutrients is a multi-stage process associated with digestion and absorption. During digestion, food particles break down into smaller components, which will later be absorbed by the body.
The digestive system is one of the largest organ systems in the human body. It is responsible for the processing
of liquid food. The cells of the human body require any wide range of chemicals to support their metabolic
activity, from the organic nutrients used in fuel to water that sustains life at the cellular level. The digestive
system is not only chemically effective and reduces the food compounds into their basic building blocks but
also works to keep the water from excrete digestible substances. The functions of the digestive system can be summarized as follows: ingestion (eating food), digestion (breakdown of food), absorption (extraction of
nutrients from food), and its needs (removal of waste).


The digestive system is composed of a group of organs that form a closed pipe-like structure called the digestive tract (GI) or the lymphatic canal. For your convenience, the digestive system is divided into the upper digestive system and the lower digestive system. The organs that make up the digestive system include the mouth, esophagus, stomach, small intestine and colon. There are also several accessory organs that secrete various enzymes into the digestive system. These include the salivary glands, liver and pancreas.
Because the gut microbiota contributes to the host of nutrition, health and behavior, and the composition of the bacterial community varies according to the host's phylogeny, collective evolution is considered an essential mechanism in the formation of the host system. However, this study is not ideal for examining this issue. Most studies of gut microbiota are performed in controlled settings, but the composition of the intestinal microbial community is strongly influenced by environmental factors. Really explore the coevolution.
Relations between bacterial communities in the digestive system and their mammalian hosts were Shown to provide valuable benefits to hosts 1-3. A classic example of these are found In the residue of cumin leaves, where the plant digestion allows conversion of the plant fibers into the chemical Compounds, which are then absorbed and digested by the animal [28,29]. This process is very Is of great value for humans, since it allows the use of solar energy stored in the fibers of the plant by converting them Into food products, such as milk and meat. Thus, microbiota in business humans has been studied In detail. However, the role of microorganisms in other parts of the digestive system (GIT) Such as the large, small intestine, has received little attention. As a result, our understanding The microbiota typical of different parts along the GIT is still very limited. Recently, two studies examine the intricacies of the microbial digest communities in the lore iron and steer the composition Of microbiota digestion and mucosa-associated in GITs of pre- weeded6.6 carts. However, there is little knowledge on the microbial communities of GITs of beef cattle, particularly Holstein cattle, which Now known as the highest production milk animals in the world.
Little is known today about "microbialization" and colonization populations of small intestine of sheep, despite their expected vital role in host metabolism, immunity physiology [30]. There is a reports the first characterization of the microbial composition of fecal functions, obtained through the implementation of a multimodal strategy. The optimized protocol was first invented for DNA extraction and amplification from sheep fecal samples.Then, a 16S rDNA sequence, a methanol mix rifle and a rifle metaproteome was applied to unravel the taxonomy, potentially genetic functions being actively expressed in the respective tracks. In functional terms, we identified 2097 gene and 441 protein families, finding that special fecal microbiota was mainly involved in catabolism.We tested transport carbohydrates and degradation activities identified specific pathways, such as methanogenesis for Euryarchaeota and acetogenesis for P. In addition, our approach has allowed identification of proteins expressed by a eukaryotic component of the microbiota. Together, these findings reveal the structure and function of distal gut microbiota in sheep, and open the way for further research aimed at clarifying its relationship with management and diet variables on the farm the sheep.
The taxonomic composition of prokaryotic microbiota according to the results of V4- MG, S-MG and MP are shown in Figure. As expected, raids and Bacteroidetes made over 80% of all bacteria in all cases. Furthermore, raids have been identified as the most represented lawsuit in all animals with all approaches, followed by Bacteroidetes . However, the average Firmicutes-to-Bacteroidetes ratio (F / B) ranged from 6.0 for V4-MG to 1.6 for MP, through 3.4 for S-MG. Archaeal Euryarchaeota was the fifth, seventh and third most abundant according to V4-MG, S-MG and MP data respectively. While firm levels were well maintained among people (CV <10% with all approaches), higher variation can be seen as another essential phylum, especially for Actinobacteria and Verrucomicrobia. When comparing our data with existing studies reporting on metagenomic analysis of faecal samples of other tumors, we will find a general dominance of raids on Bacteroidetes in cattle, with Ruminococcaceae and Lachnospiraceae being the most representative bacterial families of a former class (Durso et al., 2010; Shanks et al., 2011; Kim et al., 2014), in accordance with our results obtained in the sheep.
Bacteroidetes, on the other hand, was the most common component of the sheep novel according to the literature [31,32]. Consistently, a significant increase in F / B ratio from the rumen to colon was recently described in the cow [33]. Among the minor phyla, we found an exceptional amount of functionally active Spirochaetes, mainly belonging to the Treponema type, particularly T. saccharose-phylum, previously described as a large current pectinolytic spectchaete [34]. This syndrome was recently seen as fourth in the highest incidence of the microbiota of the gastrointestinal tract.
The composition of the bacteria community function in the digestive systems (GITs) of the milk The controller is fundamental, since it can affect milk formation and host health. However, our understanding of bacterial communities in the GITs of dairy cattle is still minimal. This research Analyzed bacterial communities in ten separate GIT sites (digest and mucosa of the rumen, reticulum, omasum, abomasum, duodenum, jejunum, ileum, cecum, colon and rectum) with six milk Beef. The study noted 542 types of 23 phyla distributed throughout controller GITs, With firms, Bacteroidetes and Proteobacteria controls. In addition, the data were exposed to Significant spatial heterogeneity in the composition, diversification and abundance species distribution of GIT Microbiota. Furthermore, the study concluded significant metagenomic differences predicted Profiles between GIT zones. In particular, the relative abundance of genes involved Carbohydrate metabolism was overrepresented in digest samples of fore-stomaches, and Genes associated with the metabolism of amino acids are mainly enriched in mucosal samples. In general, This report presents an insight into the microbiota GIT composition in a milk controller, and It may serve as a basis for future studies in this area.
https://www.slideshare.net/QasimMashood/digestive-system-of-camel-65670378
The belly of the camel [35] is enormous, running behind the chest, and most of it is a huge bag of 100 liters of rotten vegetation, sometimes called the rumen.
(The rumen, also known as a paunch, forms the larger part of the reticulorumen, which is the first chamber in the alimentary canal of ruminant animals. It serves as the primary site for microbial fermentation of ingested feed. The smaller part of the reticulorumen is the reticulum, which is fully continuous with the rumen, but differs from it with regard to the texture of its lining.). The heat generated by the fermentation is so great that the novel must be placed immediately below the abdominal muscles to let heat dissipate out. The contents are repeated again and again by waves of muscle cramps, and from time to time, part of the green group is pushed back into the mouth, the ground between the teeth, soaked with saliva and sinking again down. Often, in the evening, the only sound that interferes with the silence of a desert camp is the sound of camels caressing migration. The novel is not a stomach, because it has no glands and does not secrete enzymes. Only saliva has enzymes. The novel is fermented fermentation tool with bacteria and protozoa that break cellulose and assimilate it while dumping volatile fatty acids as the final product of their energy metabolism. Since vertebrates did not develop an enzyme to digest cellulose, the camel was happy to leave it to microbes. The camel only needs to cut the vegetation during the day and then mop up and contemplate the evening leisure. It takes up to 50 hours to fully process the content of the rumen. Evolution has never developed a fast way to digest cellulose. The camel has not yet earned its efforts. It simply provided a haven for bacteria. But now their time is up. The fine slush is crushed into the real belly and blown with enzymes to break down the bacteria. The stomach should not be large; Most of the hard work has already been done. The products of this double digestion, first by bacteria and then by the abdominal enzymes, are transferred into the small intestine where the nutrients are finally absorbed. Cellulose and all dietary starch were all converted to volatile fatty acids which are the main energy source of the camel. Sugars simply can not do this so far without conversion to fatty acids. The bacteria also provide the camel with its main protein source, which is converted to amino acids by the special enzymes of the small intestine. In a final transformation of the camel's digestive system, any nitrogenous substance converted to urea, usually secreted by the kidneys in a less rigorous animal, is re-absorbed in blood and transferred to the saliva to be absorbed into it. rumen where the bacteria convert it back to the protein. No wonder camels smile so smugly. The rest of the waste is dried in the large intestine and finally expels balls, dry enough to be used immediately in a Bedouin fire to make the evening bread. With such a strong digestion system, the camel can survive on shrubs that starve to starvation of other animals. However, given the odds, they graze spiders and browsers, prefer fresh shoots and leaves. They have large lips that can be searched and understood; A series of scissors on the lower jaw that can grow the plant stems on the upper palate; Large dogs for grip and broken branches; And a hard palate to crush thorny plants; While dangerous toxins are often neutralized by bacteria in rumen.

The current microbial diversity in the feeding of feedable animals facilitates the digestion of plant fibers. In this study [36], a bacterial analysis of the hunting rifle of bacteria that adhere to plant fiber in the camel was done to identify the main species contributing to the reduction of lignocellulose and volatile short chain fatty acids (VFA). The density of genes in metagenome-encoding glycoside hydraulics was estimated to be 25 per Mbp of assembled DNA, which is significantly greater than what was reported in other sources of methanomas, including the cow novel. There was also a significant representation of encoding sequences in scaffoldins, dockerins and cohesins, indicating the potential of "cellolosome"-mediated lignocellulose degradation. Binning of the metagenome assembled enabled the definition of 65 high quality genome bins which showed a high variety for degrading lignocellulose enzymes. Bacteroidetes-related species showed a high rate of genes for degrading oligosaccharide enzymes, while those belonging to Firmicutes and Fibrobacteres were rich in cellulases and heiciculases, so these dendrites were probably the key to ensuring degradation of lignosaculose. The presence of many "polisaccharide exploiting loci" in the genome Bacteroidetes indicates their broad substrate specificity and the potential for high carbohydrate reduction capacity. Analysis of the VFA biosynthesis pathways showed that the genes required for the synthesis of acetate existed in a variety of species, except for Elusimicrobiota and Euryarchaeota. The production of propionate, exclusively through the succinate pathway, was carried out by species belonging to pylea Bacteroidetes, Firms, Spirochaetes and Fibrobacteres. Butyrate is created by the butyrylCoA pathway: acetate CoA- transferase by Bacteroidetes and Lentisphaerae, but usually through a butyate kinase pathway of species. The analysis confirmed the camel's microbiology as a dense but still unexploited enzyme, with the potential to be used in a variety of biotechnological processes, including biofuels, fine chemicals and the food processing industry.
Digestion of Grass by Camel
1. Camel is Pseudo Ruminant. Pseudomonas is an animal that eats large quantities of tumors but does not
have a four-cell stomach such as chalk.
2. The digestive system consists of the organs directly involved in the absorption and digestion of the food,
its passage through the body, and the expulsion of the non-absorbed part. These organs are usually grouped
under two heads 1) alimentary canal 2) accessory organs
3. The drainage channel is a tube that extends between the lips and the anus. Mouth, pharynx, esophagus
stomach, small intestine and colon
4. Mouth: The mouth is the first part of the lymph canal. It is stretched by the cheeks, by the hard palate,
physically by the mandalian's body and behind the soft palate. Function: Chewing (with the help of teeth)
and lubrication of feed.
5. The pharynx: The pharynx is the part of the digestive tract that receives food from the mouth. Two
branches of Pharynx Esophophagus: which carries food on the stomach Trace: trachea, which carries air to
the lungs. The camel does not have guttural bags.
6. Belly: The belly of the camel consists of three different parts. The biochemical pattern of microbial
fermentation is generally similar between tumors and camels. C1: A large cell is divided by a robust transverse
ridge and divided into the cranial tail. C2: is a relatively small fraction and is not entirely separated from C1
C1: which originated from C2, located on the right side of C1, C3 is a long, organ-shaped intestine tube.
7. The intestine: The camel's intestine has the common name of the large, small intestine. The small intestine
has a length of about 65 meters. Neutralizing: Bicarbonate ions from the pancreas and cheating from the
liver neutralize the stomach acid to create a pH environment suitable for pancreatic enzymes and intestines.
Digestion: enzymes from the pancreas and lining of the small intestine complete the breakdown of food
molecules. Bile salts from the liver emulsify fats.
8. Absorption: Round folds, Willi and microvilli enlarge the surface. Most nutrients are absorbed actively
or passively. The colon has a length of about 78 meters. Absorption: Semi-proximal of the colon absorbs
salts (eg, sodium chloride), water and vitamins produced by bacteria. Storage: The distal portion of the colon
holding feces until it is eliminated.
10. Liver: The liver is the largest gland in the body. Bile salts from the liver emulsify fats. 5.Pancreas:
Bicarbonate ions neutralize gastric acid to create a pH environment suitable for pancreatic and intestinal
enzymes.
11. In camels, two necessary contraction sequences (A and B-contractions) were observed in A- sequences
begin with a contraction of C2 followed by a contraction of C1 tail about 4 seconds later. B- sequences begin
with a contraction of cranial C1 followed by C2 Caudal C1. B- sequences last about 9 seconds. The digesta
flow through the channel between C2 and C3 occurs during the contraction of C2.
12. After shrinkage of cranial C1 content is pumped into the esophagus. This is followed by an antiperistaltic
wave towards the oral cavity. Equation of gas from the abdomen of camels, occurs during the contraction
of the C1 tail. During eating and rampant frequent abdominal motility (about 100 A and B- sequences per
hour).
13. Rumen saliva lorica on microorganisms kill carbohydrate digested pharnx digestion C3 C2 C1 mixing
activity antimicrobial saline saline juice pancreas sequence A sequence slow attenuation neutron neutron
neutron on ion absorption digest storage of waste
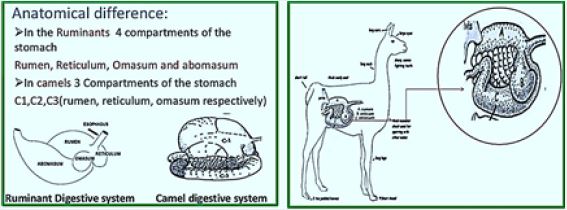
14. The anatomical difference the histological difference
15. Anatomical difference: in Ruminants 4 cells of the abdomen Rumen, reticulum, Omasum and abomasum
in camels 3 cells of the stomach C1, C2, C3 (rumen, reticulum, omasum respectively)
16. Continue ... Cell 1 is not as phony as the novel. Cell 2 is not arranged by the structure of the honeycomb
of the reticulum. Cell 3 is not pelleted and filled with laminae as life.
17. In adult camels the small intestine is 65 meters in length, whereas in the case of cattle is the small
intestine is 20 times longer than the length of the animal. Example: A controller, 2m long and has a small
intestine of 2 × 20 = 40m long.
1. Histological difference: In camels, unlike tumors, only the back parts of C1 and C2 are made of squamous keratin.
Among rumen bacteria, bacteria play important roles in the biological degradation of their plant fibers due to their large Biomass and high activity. To maximize the utilization of fiber components such as cellulose and hemicellulose by animals, the disease The ecology and functions of rumen bacteria should be understood in detail. Analyze the recent genome sequence of representative fibrolytic Bacterial species discovered that the number and variety of digestive enzymes in the plant fiber is clearly different from fibrobacter sosinogenes and ruminococcus flowrance.
Therefore, the mechanism of plant fiber digestion is also considered different among these Two species. The ecology of individual microbial fibrolytic bacteria has been studied using pure electron microscopy cultures. Recently Advances in molecular biology techniques complement the shortcomings of conventional techniques and allow an accurate assessment of The ecology of specific bacteria in a mixed culture, even in situ and in some form. Molecular monitoring of fibrolytic species in bacteria rumen pointed to the dominance of F. succinogenes. Nutritional interactions between fibrolytic and non-fibrolytic bacteria are important Maintaining and promoting fibroblastic activity, particularly in terms of crucifixion of metabolites.
Recently 16S rDNA-based surgeries Show that fibrolytic species are currently recognized as F. succinogenes and two species Ruminococcus with fibrolytic activity (the ability of some proteolytic enzymes to dissolve the fibrin in blood clots facilitating wound healing. See also fibrin.) may Represent only a small fraction of the fibrolytic population and that unultured bacteria may be responsible for digestion in fibers The novel. Therefore, the characterization of these identified bacteria is important to understand the physiology and ecology of the fibers Digestion. To achieve this, a combination of conventional and modern techniques can be useful.

Meeting the Increasing demand for food, food that is produced in the agriculture. The UN expects the total Agricultural production (including crops and livestock) should be 60% higher than in 2005. With animal protein According to estimates, demand is rising at a relatively rapid pace Because global meat and milk production will have to rise in 76 and 63%, respectively. Livestock relies mainly on Ruminant animals. Cattle, Sheep, Goat are the main members of this family of the livestock.

The rumen is a complex ecosystem composed of anaerobic bacteria, protozoa, fungi, methanogenic archaea and phage. These bacteria interact closely to break down Plant material that cannot be digested by humans while providing metabolic energy. The host, in the case of archaea, produces methane. As a result, rumors Produce meat and milk rich in protein, vitamins and minerals of high quality, and thus contribute to food security [39].
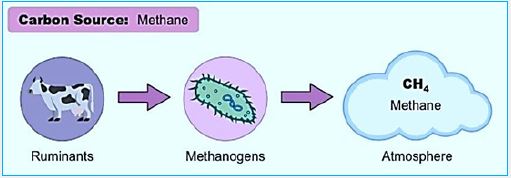
Methanogenic Bacteria
The enzymatic conversion (hydrogenation) of CO2 to methane (CH4) is one of the fundamental
transformations of life, very much known from the digestion of vegetation in ruminant food management.
The methane gas produced by seep in new Zealand pollutes the upper atmosphere and brings about the
warming of the earth. The gases that protect the earth's heat are called greenhouse gases. They include
carbon dioxide and methane. In recent times human activity has established the balance of these gases in
the atmosphere. Cars and factories produce carbon dioxide, as does burning forests. Farm stock such as cows
and sheep produce methane by belching, and many farms in New Zealand [40] produce vast amounts of
methane gas. All the extra carbon dioxide and methane in the atmosphere hold much more heat. This causes
global warming. If it continues, life as they know they will be in danger.
About 200 genes are required by methanogenic bacteria to carry out this biochemical process [41].
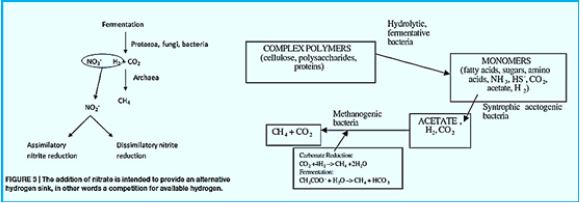
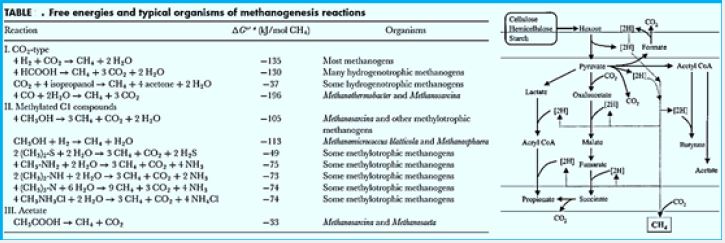
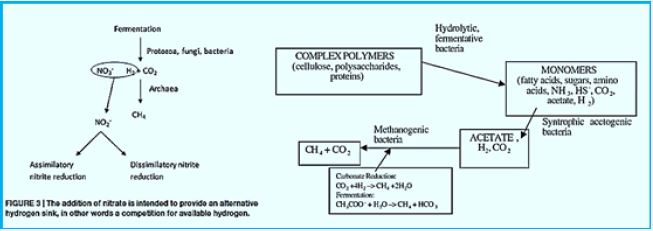
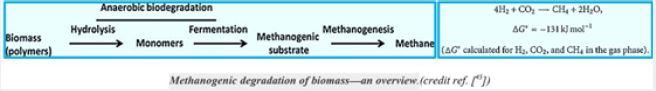
Methane can be produced by a thermogenic or abiotic formation that occurs in carbon nanotubes beneath the surface or by a biologic or biogenic formation that usually occurs on or near the Earth [42]. Biogenic CH4 is the result of complex biochemical reactions by bacterial groups during the decomposition of organic matter in anoxic environments. Three different tropical groups of bacteria are needed for methanogenesis (Fig.). These include
1) hydrolytic fermentation bacteria,
2) synthetic synchronic bacteria, and
3) methanogenic bacteria [43]. Fermented bacteria initially produce hydrolysis of complex organic compounds
for acetate, longer bound fatty acids, CO2, H2, ammonia (NH3) and hydrogen sulfur (HS-).
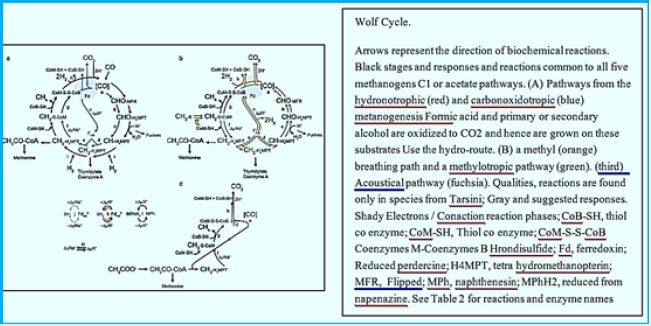
Despite the limited metabolic range, archaea are either melodic or pleasing Phylogenetic and ecological diversity. Only three types of pathways are known: CO2 reduction, methyl group reduction and acetylenic response. They are civilized grouped into five irregularities based on their phylogenetic properties and phenotypes. In addition, non-cultural exhibits that may represent new orders exist in many environments. ecology mediators emphasize their complex interactions with other anaerobic and physical-chemical agents that control their functioning [44]
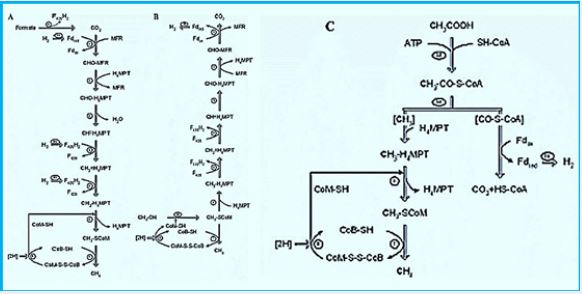
Blankets: Fred = Reduced form of brevetoxin; Fox = oxidized form of peroxide; F420H2 = reduced Form Coenzyme F420; MFR - Manifold; H4MPT = Tetra hydromethanopterin; CoM-SH = Coenzyme M; CoB-SH = Coenzyme B; CoM-S-S-CoB = CoM and CoB; Coash Coenzyme A. Enzymes: Formal- MFR dehydrogenase (Fmd); 2. formyl-MFR: H4MPT formyltransferase (FTR); 3. H4MPT cyclohehydrolase (March); 4. Methylene-H4MPT dehydrogenase (Hmd); 5. Methylene-H4MPT reductase (Mer); 6. Methyl-H4MPT: Methyltransferase HS-CoM (Mtr); 7. Methyl-CoM reductase (Mcr); 8. heterodisulfide reductase (Hdr); 9. Format of dehydrogenase (Fdh); 10. Energy conservation Hidrogenase (Each); 11. Hydrogen hydrogen to reduce F420; 12. methyltransferase; 13. Acetate kinase (AK) Phosphotransacetylase (PTA) system in Chinese Minerals; Acetyl-CoA synthesis that creates AMP In Methanosaeta; 14. CO dehydrogenase / synthy-CoA synthase (CODH / ACS).
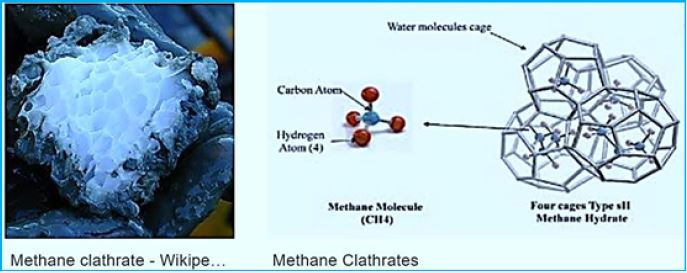
Methane is to find in abundance on earth , see on table 1. showing the 2008 estimate for methane on earth. A considerable part of it is the product of the methanogenic bacteria applying the endothermic hydrogenation reaction of converting CO2 to CH4. This methane forms. Among others, the methane hydrates clathrates on the ocean floor. The energy conserved in the high seas methane deposits minis a winning goal to fossil fuels searching enterprises.
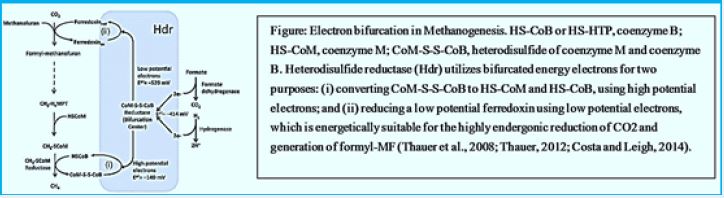
The reduction of CO2 to formyl-MFR is endergonic and driven by ion gradient via the membrane-bound energy conserving hydrogenase [5].
The activity of synthetic acetogenic bacteria is too essential to complete the agitated fermentation. Low molecular weight carboxylic acids, such as propionate and butyrate are conventional products of fermentation bacteria. However, these acids can not be eliminated by methanogens. The endogenous pathogenetic bacteria oxidize these acids to acetate (and CO2 in the case of propionate) that produce H2 or formulations as a reduced product. Therefore, the primary substrates used by the fungal bacteria to promote their growth include hydrogen (H2), CO2, acetate and form. Modangens are a group of strictly anaerobic bacteria that require reduction of environments (redox levels <-200mV) for growth and produce methane as the final product. The most common and almost universal condition for methane production is the reduction of CO2- mediated CO2 reduction (Figure), although the primary source of methane in most active decomposing environments, such as anaerobic digestion and freshwater sediments, is acetate (fermentation path, Figure) [45].
The reduction of CO2 to formyl-MFR is endergonic and driven by an ion gradient via the membrane-bound energy-conserving hydrogenase.

Methane production routes in Rumen. The bacteria that ferment in carbohydrates and in protozoa The rum produces CO2, H2 and Volatile fatty acids (VFAs). It is known that CO2 and H2 are primary precursors for CH4; Formats are also a Precursor to CH4. Methane production from Evaluation of the formats accounts for about 15- 20% The total production of methane in the atom [47]. Precursors of methane Production is converted to CH4 by methane production Archaea, from the pleasures that appeared on the earth about 3.5 x 109 Years ago or earlier [48]. We are in the suburbs Romanthus, from a mobile microbium, from Tonsarina mazei, methanosarcina barkeri and methanobacterium Formicum was isolated from the taste by Cultivation [49]. Biochemical studies of Modified melanogenesis showed that M. ruminantium, M. formicicum and M. mobile use H2 / CO2 and crystallize into Produce methane. On the other hand, M. mazei synthesizes Methane, methanol and methylamine. M. Berkery uses H2 / CO2, acetate, methanol and Methylamine for methane synthesis [50]. It is assumed that methyl coenzyme-M reductase (MCR) is It is common in emoticons, because of the last step of Methane production by methanol catalysts by MCR [51]. Moreover, since they are meddling Contain the fluorescent compound F420 (Coenzyme 420) Direct observation by fluorescent microscopy Possible, and reveals the interaction between plant levels And melodies, which connect themselves to the continuous cell Surface and receive hydrogen from it [52]. There are polymerase chain reaction-based techniques (PCR) Developed to detect the pleasures of the meal Without cultivation. The small subunit rRNA gene (SSU) (16S rDNA) types are most useful for this purpose [53].

Methane is produced by methanogens in anaerobic conditions (without oxygen) in both animals and in manure. However, when the manure usually applied to fresh soil, the anaerobic conditions necessary for the production of methane are harmed. Therefore, cattle manure accounts for only about 5% of the emissions.
The largest source of greenhouse gas (sometimes abbreviated GHG) in the production of beef comes from methane produced in the digestive tract, accounting for more than 60% of all emissions.
In Ruminants, methane (CH4) is a natural byproduct of digestion and nutrients in the intestinal tract and is created by reducing carbon dioxide through the addition of hydrogen in an highly endotermic series of events. If methane production is stopped without the removal of alternative hydrogen, the efficiency of cattle production can be impaired. Identifying technologies that reduce methane production without adversely affecting efficiency has proven to be a significant challenge for the scientific community. However, it is possible to reduce the emission of methane acid by adding certain foods, including cereals or oils, to the diet.
A global research effort has identified technologies that can reduce methane. Increasing carbon value will increase the use of these technologies in the production of a controller.
Agriculture accounted for 9% of the US GS emissions in 2014. The emissions in this sector derive from agricultural activities such as growing crops and livestock. Animals produce methane as part of their digestion. The process is called anomalous fermentation and represents almost 30% of the emissions from agriculture. Live animal manure also contributes to the emission of methane and nitrogen. Garbage management accounts for about 14% of total gas emissions in this industry in the US.
The commercial and residential sector accounts for 12% of the US greenhouse gas emissions in 2014. These include all commercial homes and businesses (excluding industrial and agricultural activities), which are caused by combustion of fossil fuels for heating and cooking, waste and waste management, and household and business refrigerators. Emissions from natural gas consumed for cooking and heating constitute about 81% of the emissions in this industry: organic waste sent to methane-emitting dumps, methane-andnitrogen effluents, and refrigerant leaks emitting fluorine gases.
Grazing: and Herbivores
The only way that grass can produce food is through pastures mainly by the ruminants: cattle, sheep and
goats - on them [54]. Most mammals, including humans, can not digest grass, or rather, cellulose. But rumor
has several cells in their stomachs. One, the Romema, contains microbes that can digest grass. The problem
is that this bacterial digestion process also produces greenhouse gas methane as a by-product [55]. Methane
is a powerful greenhouse gas, if short-lived. Has been given a potential warming rating of 25 times that of
carbon dioxide for 100 years, although it has a life of 9 to 12 years in the atmosphere.
Grass includes pasture native species introduced; It includes the campuses of South America, the North American steppe, the Saharan steppes in Africa, the southeastern Europe, Asia and North America and the savannas of Africa, Australia, South America and South and North America. Grass grass is not suitable for cutting because of poor soil, low rainfall or topography. Ants produce protein from plants in areas unsuitable for other agricultural activities. And before there were modern cattle there were wild rumors: bison or buffalo, karibah, cherry, muffler, orch and goats. And they all produce methane. The large herds of bison that were scattered across the steppes in the United States before the white settlement, along with other native rumors such as reindeer and reindeer, are estimated to have produced 86 percent of the current US herd of cattle [56].
Ruminal bacteria involved from the area of goat were incubated with nitrate or nitrateIn a growth medium
containing starch and cell. The nitrate reduction rate was 2.5times faster Of the nitrite reduction rate,
which led to nitrite accumulation. The increase in the nitrate increased the peak concentration of nitrite
and extended the time until the nitrite disappeared [57]. The addition of nitrate decreased in methanogens
in part by the consumption of electrons, but to a greater extent Largely by suppressing fermentation due
to accumulated nitrite. Addition of mucosa Increased the rate of nitrate reduction, and to a greater extent
increased the rate of nitrate reduction, Which facilitated the negative effect of nitrite on fermentation and
cellular digestion. As a result, A combination of pomrat and nitrate decreased from methanogenesis without
fermentation suppression It is the energy consumption in the transformartion of CO2 to methane (CH4)
that lcurrently limuits efficiency of feed to meat or milk,. Scientists are exploring food additive to improve
thew situation.
If nitrate intake conditions [58] are maximal conversion of ammonia nitrate and reduced methane production limit the amount of nitrate that can be fed, then an alternative approach is to use nitrate in combination with other strategies known as reduced methane emissions. There has been almost no research into strategies to reduce methane emissions with different mechanisms. [57] found that the use of pumarate and nitrate was beneficial, and the addition of nitrate and pumarate did not affect nutrient intake, microbial protein supply, and blood profile [59], found that combining methane inhibitors with low-dose complementary mechanisms may be more effective and effective in reducing methane emissions from computers without compromising food digestion. "A combination of saponins and nitrate may be such a practical strategy, [60] showed in vitro combinations of nitrate with saponins and sulfate suppressed methane production in an additive manner, with a maximum reduction of emissions (nearly 46%) observed in a combination of three inhibitors 0), the effects of sulfate and nitrate on methane production were additions, indicating the potential for this combined approach. Of course, there is an experiment to perform long-term performance with large numbers of animals to better assess the persistence of single strategy approaches or a combination of methane consumption for methane reduction, performance, meat and milk.
Methane losses (CH4) reviewers fed a variety of diets are documented throughout the literature [61,62]. However, the use of wet distillers grains with solubles (WDGS) effects on CH4 production in the controller Not well defined. Previous research results have yielded Proof of evidence of feeding effect WDGS on CH4 emissions [63]. Report Reduction while [64] watch the enlargement and [65] no WDGS effect found on enteric CH4 emissions. Our hypothesis was that at that time High concentrations in food diets (> 30% DM), WDGS Will increase CH4 production. Therefore, our goals were To measure the effects of WDGS inclusion in the underlying steam-flaked corn (SFC) Nutrition on energy metabolism, balance between C and N, and CH4 enteric losses a visitor, and to quantify nutrition NE value for WDGS.
Most of the world's agricultural lands are grassland. For reasons of rainfall, soil type or topography it can not
be plowed and never watered. The only way to produce food from grassland is grazing ruminants - animals
like cows, sheep and goats - on her. Most mammals and this includes humans, Can not digest grass. But
ruminants have several cells in their stomachs. One, the ruminants, the microbial "homes" that can digest
grass. The problem is that this process of bacterial digestion, too produces methane (CH4) the greenhouse
gas as a by-product. Methane is a powerful greenhouse gas, if short-lived. It gets a potential global warming
rating of 25 Times that of carbon dioxide, although it has a life of 9 to 12 years in the atmosphere, compared
to with carbon dioxide that can last more than a hundred. Some segments of the community jumped on this
information, claiming that they ate less beef, or not Eating it at all will be better for the environment. But it
raises a few questions: What happens to the grasslands that are no longer grazing? What do you eat instead?
How would you compare her carbon footprint? What about the role of animals Traditionally play farms, eat
waste and provide natural fertilizer? And should we compare the cycle of natural cattle - which actually fixes
carbon - with the burning of fossil fuels? Grassland accounts for 60 percent (!) of the world's agricultural
land. Can we ethically refuse to produce food Of so many lands in a world where a hungry population is
growing rapidly? Ants produce protein from plants in areas unsuitable for other agricultural activities. And
before there were modern cattle there were wild rumors: Bison or Kariba, Cherry, Flop, ornament and goat.
And they all produced methane (!).
Does the Cattle Gut Microbiome Affect Methane Output?
In the past, studies have linked nutrient efficiency to methane production , according to which a more
efficient feeder controller produces less methane [66]. A group of bacteria located in the mango area, known
as methanogens, is responsible for producing methane. Methane production, or methanogenesis, is an
energy - inefficient process in which an estimated 6-12% energy consumption of an animal is transferred
to methanol-genesis. This energy can otherwise contribute to the performance of animals such as growing
or producing milk.


Abundances and Flux Estimates of Volatile Organic Compounds from a Dairy Cowshed
Animals are responsible for 18 percent of greenhouse gases that cause global warming, .more than cars,
airplanes and all other forms of transport produced together This does not mean you can ignore methane
- lowering methane levels is definitely a goal to continue. The good news is that since the early 1990s, the
trend in methane production has slowed down and has even improved in recent years.
Climate change is caused directly or indirectly by human activities that alter the composition of the global atmosphere that constitutes a blanket of gases on Earth. Long- term production of these gases makes the natural climate bread more than usual. The gases include:
1. Carbon dioxide (CO2), which is the gas derived from organic and industrial firewood, industrial engines
and vehicles;
2. Nitrous oxide (N2O), gas and fertilizers exposed to solar heat;
3. Methane (CH4), a gas produced mainly under anaerobic conditions such as those that occur when animal
by-products are fermented or rice horses are introduced for full water coverage;
4. Ozone (O3), aerosol gas spray like perfumes, cosmetics, household sprays; and
5. Water vapor from open, natural water bodies such as lakes and oceans.

Livestock, particularly ruminant animals, produce in their rumen the energy-rich gas methane in massive amounts. Cows can look quite benign when they chew grass in agricultural fields, but some environmentalists warn that flaming, hiccuping and shackling of animals is a significant contributor to climate change. A 2006 UN report [68] estimated that cows, along with other animals such as sheep and goats, contribute about 18 percent of the greenhouse gases that warm the planet - more than cars, airplanes and all other forms of transport that they have assembled. Because methane-rich controller emissions, gas is 21 times more efficient than carbon dioxide when capturing heat in the atmosphere.
Nowadays, this treasure is exerted to the atmosphere and causes a part of global warming. The production of this gas (methane CH4) is reducing milk and meat production up to 15% (!). The "Big-Cowshed" idea can assist in the harnessing and harvesting od the methane gas. However, the technology to do that has not been identified yet.
The increase in the world's human population will reach 10 billion people in our time. The feeding, the production of food and at the same time the maintaining of an environment that is livable for humans, is one of the significant challenges of humanity. Fortunately, agriculture relies upon a great extent on the cultivation of ruminant animals that are a source for proteins in the form of meat and milk and are using grassland as feeding location, places where otherwise could not support any other form of agriculture. However, the biological process of utilization of grass, the digestion of cellulose, is carried out by bacteria that dwell in the intestine system of the ruminants and those produce as by-products methane gas and other compounds that when not used properly, can become an environmental hazard. A significant challenge for biotechnology is to find the way, the technology to harness and harvest these potentially hazardous materials, and use them correctly for the benefits of mankind.
Intensive farming of animals has been widely adopted to meet the demands, such as milk and meat, of a growing human population. The largest of these facilities, called concentrated animal feeding operations (CAFOs), are undoubtedly profit-driven companies. Although history is associated with rural farmland, it is often located near urban areas. The growing number of animal facilities and their proximity to human settlements have led to increased odor complaints from neighbors. Th has been undertaken to conduct olfactory identification measurements and to implement possible abatement strategies [70-73]. The significant odors are amine, sulfate, phenols and volatile fatty acids (VFAs).
Animal husbandry and manure management were documented explicitly as a significant source of methane, ammonia, nitric oxide and particulate matter. Even though Volatile organic compounds (VOCs) are also produced, a lot Less information exists regarding their impact. We report the Mass spectrometry of chemical ionization and acoustic photography Spectroscopy measurements of VOC mixing ratios over an A measurement period of 2 WK in a large barn in the Federal Reserve agricultural Research Center (FAL), Marianne, Germany. The high resolution of these measurements allows this An insight into the sources of typical animal emissions Setting up management. Feeding hours and stable manure Remove, large spikes of the mixing ratio of the number of VOCs were Observed and coordinated with methane and carbon simultaneously Dioxide and ammonia improvements.
The methane gas, which was released by 90 gas cows [75], exploded in a warehouse in Germany, hit the roof and injured one of the animals, the local police said. In a statement, the force said that high levels of methane gas had accumulated inside the building in the central German city of Rasdorf on Monday thanks to the belching of animals and the gust of gas, before "a static charge caused the gas to burst into flames." The subsequent blast hit the roof of the cow shed, Reuters reported.
Emergency services that arrived at the scene made fuel calls to check for additional explosions.
Animals can emit up to 500 liters of methane of greenhouse gases every day through belching and rough mood. Cows also release large amounts of ammonia.
Finnish Dairy is using thew bug cowshed (barn) to capture methane. an estimated 2.5 percent of Finnish greenhouse gas emissions come from dairy production (Source: Statistics Finland, 2016). As a significant milk producer Finnish dairy [76] wants to find new solutions that could allow us to reduce the amount of methane gas that is generated, and to make use of it as efficiently as possible.
The separation of manure to liqid s and soklids enable a bacterial treatment oif the liquid fraction to produce methane gas from the manure [77]. The U.S. currently has just under 250 digesters on livestock farms. There are many different types of digesters. Digesters completely enclose the manure to exclude oxygen and encourage methane producing bacteria to be active. The resulting biogas can be used to generate electricity. The leftover manure (effluent) can be used as a crop fertilizer or can undergo solid-liquid separation with the resulting products being used as described above.
The composition of cattle waste depends on the conditions in which animals are kept on the farm milk Cows, cows and calves [78]. Cattle beef was removed From cow houses contains food and bedding (Straw, sand, sawdust, etc.) in addition to reliable and liquid waste of animals. Many of these solids are not perishable or slow. The waste can be collected Through mechanical or hydraulic scraping of The floor and dragging the material into the garbage pit. Separation of liquid and solid fractions of waste is an Upstream action in the treatment process: Dehydration of the solid fracture reduces the cost of the shipment And increases the energy yield in the combustion process. When separated solid fractions have TS suitable Content and VS / TS ratio, then they are suitable for the production of compost [79,80] or energy [81] If the liquid fragment is separated, the total The most significant volume, showing low solids and ammonium content, can be deployed on fields during most of the year Without causing environmental problems. After separation, The concentrated dry matter can be applied Nitrogen-rich fracture during growing crops [82]. When collecting garbage by Wash instead of scratching and dragging the floors, this is Dilute and disperse water more easily through a sieve. There seems to be a flushing operation on the sieve: the more so Fluid liquids prefer to detach delicate and colloid solids from longer fibers that are retained by the screen and pass through the sieve. Therefore, liquid filters exhibit higher solids content compared to filter garbage collected from the animal by scraping and dragging
"Science" publication [83] reveals that a study of 1000 cows conducted in four European countries was done to understand how well antimicrobial omes can be controlled by the host animal, and identify properties of the host-microbial axis of this host rum determine fertility and methane emissions. The rumen microbiome, phylogenetically linked and with a preserved hierarchical structure was identified. A subgroup of 39 members of the core created hubs in everyday occurrence networks that connect the microbial structure to host genetics and phenotype (methane emissions, harmful and blood Metabolites, and milk production efficiency). These phenotypes can be predicted from microbial biomass using Machine learning algorithms. Therefore, the network core bacteria present key targets for odor manipulation Towards sustainable and environmentally friendly agriculture.
Hosting one of the most diverse microbial communities known Man, the issue has long attracted the great interest of microbiologists. Physiologists and nutritionists also understand the central role of the weight indigestion and fiber feed and provide nutrients to the host animal. These activities allow noise to provide people With foods, especially milk and meat from the inedible plant the material, including industrial byproducts, allows many rural communities around the world to survive in the place where agriculture can be cultivated Impossible. However, there is an environmental cost in which the dead, Produce significant quantities through their Roman microbial of the greenhouse gas, methane [84].
On the basis for the genetic determinants of dry bacteria it should be possible to optimize their abundance through selective growth programs. Different, and perhaps more immediate, the application of our data could be a change in the colonization of early life, a factor that has been shown to motivate the composition of microbial organisms Activity later in life [85-87]. Vaccine and mini - core related With feed efficiency or methane emissions as precise probiotics The approach can be considered reasonable to complement heredity microbiome toward optimal drainage function. The research focused on two breeds of cow's milk, but the results are probably applicable to cattle and other ants species. Given the high importance of the diet in performance and composition of microbial ruman, these programs should take special care recognition of reasonable feeding regimes. In this framework, following the total predicted the effect of heredity identified with an identified attribute Microbes on production measures should result in greater efficiency and animal industry from a more environmentally friendly source.
The Capture of Methane Gas from the Atmosphere
The window to action in the climate closes before our eyes, and as the emissions continue to rise, researchers
ask us to consider the lesser of two evils. In a new interpretation, the authors proposed a wild idea that
would deliberately release more carbon dioxide (CO2) into the atmosphere while removing an even worse
.greenhouse gas - methane
Methane (CH4) is the second most dominant greenhouse gas, and while it is slightly less fertile than CO2, it is 84 times stronger. Converting this agricultural and industrial byproduct to more CO2 is not necessarily crazy at first.
Of course, replacing these two gases would require the removal of industrial methane as well as efficient conversion technology, none of which currently exists.
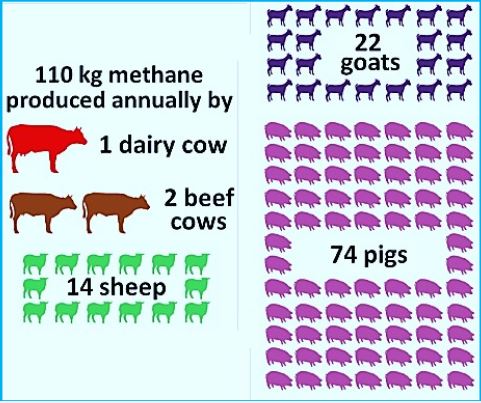
If these two obstacles can somehow be cleared, however, researchers think their idea could take 3.2 billion metric tons of methane out of the atmosphere, return .concentrations to pre-industrial levels and reduce global warming by 15 percent Methane gas is fossil fuel, it is found as a mineral in drilling, and is found in many neighboring aquifers with water, which exists in the deep sea. Animal Bees, especially ants, produce methane gas as a byproduct of their digestion process using fungal microorganisms and archaea activity that produces methane in high altitude and is operated as a gas in a feeding process. The milk cows produce about 100-150 kilograms of methane a year, this is about 500 (350grams) liter of methane gas per day.
Methane (CH4) is a major greenhouse gas, second only to CO2, and is emitted into the atmosphere at various concentrations from a variety of sources. However, unlike CO2, which has a square moment and can be captured physically and chemically in a variety of porous solvents and solids, methane is not entirely interactive and has fragile interaction with most materials. Thus, methane capture presents a challenge that can be addressed only by extensive screening of materials and brilliant molecular geniuses. Here we report a methodological method in silico studies on methane capture efficiency of two different materials systems, ie liquid solvents (including ionic liquids) and nano-crystalline sulfates. Although none of the liquid solvents are as effective as methane, a systematic survey of more than 87,000 zeolite structures has led to the discovery of a handful of candidates that has sufficient methane absorption capacity as well as CH4 / CO2 and / or CH4 / N2 selectivity to be technologically promising.
Agricultural methane has become a controversial issue as concerns about climate change and global warming have increased. Data published by the United Nations in September 2013 attributed 14.5% of all man-made greenhouse gas emissions to being linked to animal supply networks, 39% of which came from the cow's digestion process.
One area of research that begins to produce benefits is the extraction of energy from cow droppings in the process of anaerobic digestion. During the counting process, waste material, usually in the form of cattle manure, is treated and disintegrated in a closed digestive instrument for 30-60 days, when it can be used as biogas. At the end of 2013 there were about 125 such factories in Britain.
Scientist suggests a crystalline material, known as zeolite [89,90], has the potential to act as a sponge, they say, soaking up methane from the atmosphere. The porous molecular structure, relatively large surface area and ability to host copper and iron in zeolites make them promising catalysts for capturing methane and other gases. In practice, the whole contraption would look like a giant electric fan, forcing air through a series of chambers lined with zeolites or other similar catalysts. Once captured, the methane could then be heated to form and release carbon dioxide.
As is often the case with issues that intersect with energy and climate change, there is no crystal clear answer. However, with research into capturing methane directly from the cow, its deposits and also lowering the overall level offer enough green shoots to suggest that merely slapping on a tax to cull the herd would be a reductive step.
Ventilation

The service model reveals a ventilation control system for a large barn. The ventilation control system includes many sets of ventilation vents that are formed in two opposite and fitted walls, multiple ventilation ventilators arranged on the edge of the walls of the large barn, a harmful gas ventilation control device and a power supply installation. , Where ventilation vents are supplied with start-up ventilation devices; The starting devices for ventilation and ventilation fans are connected to the apparatus for controlling the harmful ventilation; The starting device for ventilation, ventilation fans, and the PIR control are plugged in with the power supply. The ventilation control system has the technical effects of automating automatic measurement regulation and scheduled automatic regulation on the air purity of the large barn, allowing the large cowshed to maintain an environmental condition conducive to growing cows, improving the survival rate, slaughter rate and input- output ratio of live cows and achieving good economic benefits More.
Methane Storage in Metal-Organic Frameworks [91]
Natural gas (NG), whose main component is methane, is an attractive fuel vehicle applications. The realization of safe, inexpensive and convenient means and materials for high capacity Methane storage can significantly facilitate the application of vehicles powered by natural gas. The adsorption-based process that includes porous materials offers efficient storage. The methodology and the pores have examined the organic metals Potential candidates because of their particularly high pores and pores / cage tuning Sizes and functional sites that are easily managed. In this view we provide an overview of The current state of metal-metal frames for storing methane.
Dissolving Methane in Mixtures of Methanol Plus Various Hydrocarbons at High
Pressures [93]
It is Know that the use of natural gas as a vehicle fuel is an advantage; However, the drawback of this
application is the storage limitations of natural gas. One method for storing natural gas at moderate
temperatures and pressures is to dissolve it in organic mixtures. This research uses a mechanism for measuring
gas solubility in liquids at high pressures. The solubility of methanol + n-hexane, methanol + n- heptan, and
methanol + cyclohexane in 303.15K and methanol + benzene and methanol + methylbenzen at 288.15K
and 303.15K were measured. The experimental pressures are up to 12.0MB. According to the new solubility
measurements there are uncertainties of 0.002 (mole assessment). Meanwhile, the volumetric volumes of
liquid phase mixtures at high pressures have been determined and well evaluated by the Aalto-Keskinen
model. Because of the polarity of methanol, the state's PRSV equation with the Vid-Vidal mixing mixture
was used to estimate the equilibrium data for methane + methane + hexane, methane + methanol + methane,
methane + methanol + cyclohexane systems and coordinate the data For methane + methanol + benzene
and methane + methanol + methylbenzene. The mean absolute deviations (AAD) of the correlation were
3.0% and 1.9%, respectively.
Technology: Acid Bath Makes Methane Easier to Handle [94,95]
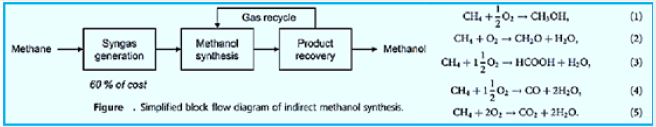
Many natural gas reservoirs can be used in remote locations in the next century, thanks to two breakthroughs in catalysis. Both systems should allow gas engineers at the sites to convert methane to methanol, a much easier, safer, and cheaper liquid for a pipeline to consumers hundreds of kilometers away. The study is reported in this week's issue of Science. The reactants are converted into a mixture of products, including carbon monoxide, hydrogen, water and carbon dioxide. The steam added in the next step changes the mixture to a hydrogen and carbon monoxide mixture called Syngas. Syngas can easily be converted to methanol with a copper catalyst and zinc oxide.
In this paper, the direct conversion of methane to methanol seems like a good idea. The trade between conversion and selectivity for products is expected and can enter Principle can be overcome by recycling of non-conversion Methane. Examples of direct industrial processes Antioxidant methane (formaldehyde) can be oxidized Can be found [96-98], but there are no examples Direct oxidation of methane to methanol, which technically requires only a change in the reactor's operation. Still, The methane-to-industrial conversion quoted was Of such a low capacity (<1 t per day) that it will be A large pilot scale machine in current terms. To Quote Arutyunov [9]:
Methanogenic Bacteria
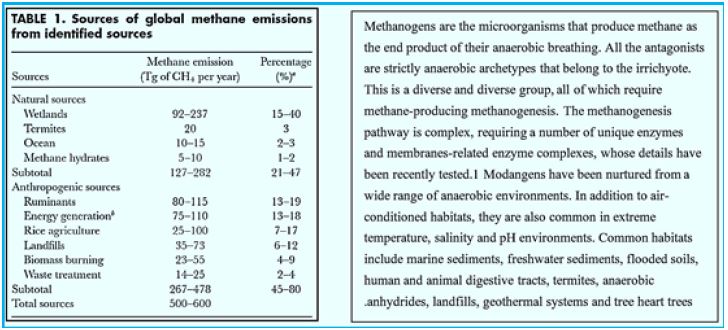
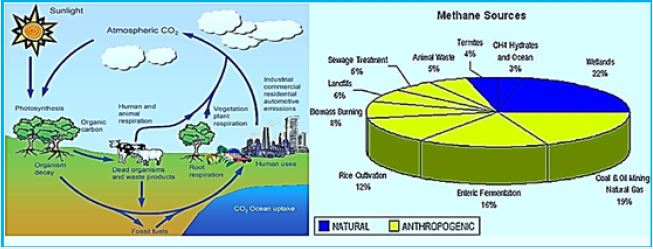
It is estimated that the world's Ruminant population [99] produces 77 000 000 tons of methane annually,
which constitutes about 15-18% (dee diagram methane sources) of all atmospheric emissions [100].
Others speculated that these were domestic animals responsible for all of total methane production; [101]
Ruminants produce bout 97% of the methane produced by domestic animals. About 75% of this production
comes from cattle, with The rest are derived from other animals, such as Water, sheep and goats. This is
given a world population of some 1.3 billion cattle, calves, it is assumed that each animal produces 44kg of
methane Every year (for 1990).
Methane-bacteria are anaerobic growing by producing methane gas. These bacteria And their exotic metabolism inspired decades of research in microbiology Continues to push the limit of what we know about how bacteria retain energy to it grow. The study of methanogens helped clarify thermodynamics and bioenergetics The basis of life has contributed to our understanding of evolution and biodiversity, and we have has led to an assessment of the social benefit of studying tropical interactions between environmental bacteria, since they are essential in the microbial conversion of biogenic methane carbon, high energy fuel. This review discusses the theoretical basis for Conserving energy by manipulators and identifying gaps in the biology of a potential oscillator Being stuffed by organisms that have not been exposed or have not yet been engineered.
The domestication of animals brought the ruminant animal to human culture, the cattle, sheep, goats
and camels. These animals support humans with food and utilize that are not suitable for intensive land
cultivation of agriculture that is based on plants. But the ruminants, as beneficial animals, have the drawback
of methane production as a by-product in the digestion of grasses and cellulose derivatives. The enormous
amounts of methane that this animal product is vast, as reported below.
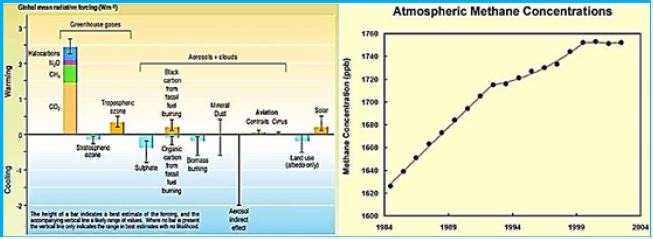
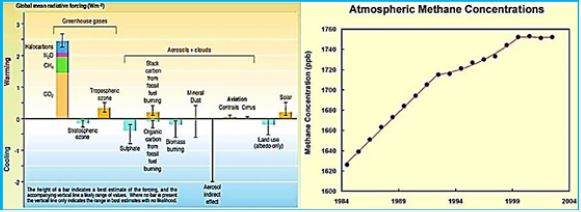
(HINDAWI)Greenhouse gases such as CO2, methane (CH4), nitrous oxide (N2O) and ozone (O3) contribute to climate change and global warming through the infrared radiation in the atmosphere [102]. CH4 is classified as a trace gas and is estimated to have a total global concentration of 1,774 ± 1.8 parts per billion (ppb), with a total increase of 11 pages since 1998 [103]. Methane is a particularly strong gas trace because of its global warming potential, 25 times that of carbon dioxide, and its atmospheric lifetime of 12 years; It is the second-largest anthropogenic greenhouse gas behind carbon dioxide [104]. Methane can also increase ozone in the troposphere of the atmosphere where the greenhouse effect occurs and increase the stratospheric water vapor, which can both add to the radiation power of the gas by about 70 percent. In general, 50-60% of methane emissions are from the agricultural sector, particularly from animal production activities; The primary source of methane is from animal sources from Mazars [105,106].
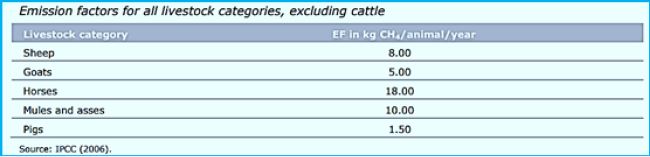
Domesticated animals, such as cattle, sheep, and goats, produce up to 86 million metric tons (Tg) of methane per year [107]. 18.9 Tg are milk visitors, 55.9 Tg are beef cattle and 9.5 Tg are goats and goats. Data from Johnson and the department estimate that the annual global contribution of methane is 6.2-8.1 , 0.9-1.1 Tg of camels, and methane production within the pig and horse axis is 0.9-1.0 Tg and 1.7 Tg, respectively.
Methane is produced in ruminants as a product of healthy fermentation of food. Although methane production can occur in the lower gastrointestinal tract, as in the sex nuggets, 89% of the methane emitted from corpuscles is produced in novels and exhaled through the mouth and nose [108]. When methane inhales into the atmosphere, it is rumored that the loss of energy derived from food is about 2-12%, depending on diet [109].
Loss of methane to the atmosphere varies depending on the species. Estimates of energy losses derived from meat, cattle and beef ranges from 5.5 to 9.0 percent, 6.0 to 7.5 percent, and 3.5 to 6.5 percent, respectively. For buffalo and camels, the loss of diet energy in the form of methane ranged from 7.5 to 9.0% and 7.0- 9.0%, respectively .Estimates of methane losses from feeders also vary based on geographical location, feed quality, nutrient intake, feed composition, and feed processing. Further discussion of the effect of dietary components on methane emissions in Section 4.1
There are large amounts of methane hydrate beneath the sea floor. Some countries hope to become independent
of energy imports by exploiting marine gas hydrate deposits near their own coasts. The technology for
production, however, is not yet available. Furthermore, the risks to climate stability and hazards to marine
habitats associated with extract ion of the methane hydrates must first be clarified.
Biogenic methane is formed in the sea f loor by the microbial breakdown of biomass. The biomass consists of dead planktonic [44,111,112] organisms such as microalgae or krill that sink through the water column to the sea f loor and build thick sediment packages over time. Methane-producing microorganisms break down the biomass into methane and carbon dioxide.
The existence of methane hydrate has been known of since the 1930s. But only in the past 10 years has it
become an object of serious consideration as a potential fossil energy source for the future. It is now possible
to project the available global amounts with some confidence. Researchers are trying to identify the highestyielding
deposits.
Most of the methane's emissions are directly related to methane-producing methane in warm and humid soils as well as in the digestive tract of individual animals. Methanogens are microorganisms that produce methane. To produce energy they use an anaerobic process called methanogenesis. This process is used instead of aerobic processes, or with oxygen, because the immune system can not metabolize in the presence of even small oxygen concentrations. When acetate decomposes in methanogens, the result is the release of methane into the environment surrounding it. Methanogenesis, the scientific term for the production of methane, occurs mainly in anaerobic conditions because of the unavailability of other oxidizing materials. Under these conditions, microscopic organisms called archaea use acetate and hydrogen to decompose essential resources in a process called fermentation. Acetate Acetate Acetate Acetate Acetate is produced during anaerobic fermentation to produce methane and carbon dioxide.
H3C-COOH → CH4 + CO2
Hydronotopic Methanogenesis - Archaea oxidizes hydrogen with carbon dioxide to form methane and water.
4H2 + CO2 → CH4 + 2H2O
While atherosclerotic methanogens and hydrogen-hydrogenase are the two main source reactions of atmospheric methane, other original reactions of biological methane also occur. For example, wax found on leaves exposed to UV radiation in the presence of oxygen is an aerobic source of methane [113]. From the beginning What does it play?
Archaea, which produces methane, is able to save energy for ATP synthesis (adenosine triphosphate) by producing methane gas. The first indication of methane Biologic gas can be produced by Alessandro Volta in 1776 which discovered flammable Gas and freshwater swamps and the hypothesis that it is derived from rotting organic matter [114]. It was not However, by 1933, melodies were first cultivated. Can be found in anaerobic pleasures Especially in low-sulfur environments such as sediments in freshwater pools. Other examples In environments that can be accommodated by animals, they are the digestive tracts of animals (eg, ants And humans), insects, marine sediments and terrestrial environments. You can isolate from a wide range of thermochemical steps, from acidophilic to alkaliphilic (pH 3.0-10.2), from thermophilic to psycho-profiling (from - 2° C to 122° C), and A master of fresh water to the loophole environments. While melanogenesis was bound to There are no reports of polymicrobial diseases, such as intestinal dysbiosis and gingivitis Of methanogens directly involved in pathogenesis through virulence or toxins [115] . To date, methanogens are strict anaerobic archaic and require methane producers. Methanol can grow by reducing carbon compounds in one (C1) [CO2 (carbon dioxide), CO (carbon) Monoxide), methanol, methylamine, and methyl sulfide], acetate or coal to methane through One of several pathways from methanogenesis [116-118]. Regardless of the substrate used, methylases M molecule is eventually reduced to methane by methyl-coenzyme M reductase Enzyme, Mac [119]. Methanogens differ from bacteria and archaea that may produce Methane as a by-product of metabolism by their binding need to synthesize methane to preserve Energy through Wolf Cycle [120]. By this distinction, all the known organisms, so far, belong to Euryarchaeal domain.
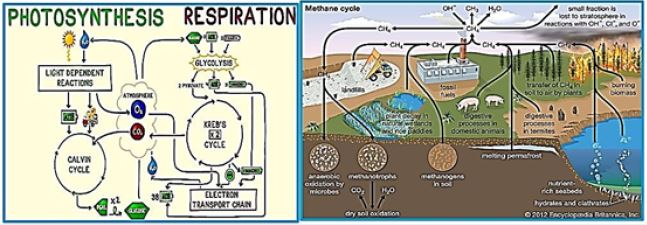
Bibliography

Hi!
We're here to answer your questions!
Send us a message via Whatsapp, and we'll reply the moment we're available!